W237809
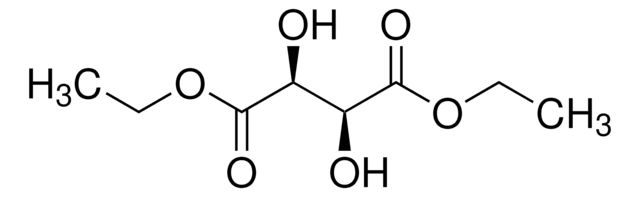
Diethyl L-tartrate
≥99%, FG
Synonyme(s) :
(+)-Diethyl L-tartrate, L-(+)-Tartaric acid diethyl ester
About This Item
Produits recommandés
Source biologique
synthetic
Niveau de qualité
Qualité
FG
Fragrance grade
Kosher
Agence
follows IFRA guidelines
meets purity specifications of JECFA
Conformité réglementaire
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 172.515
Pureté
≥99%
Activité optique
[α]20/D +8.5°, neat
Indice de réfraction
n20/D 1.446 (lit.)
Point d'ébullition
280 °C (lit.)
Densité
1.204 g/mL at 25 °C (lit.)
Application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
Allergène alimentaire
no known allergens
Allergène de parfum
no known allergens
Propriétés organoleptiques
fruity; wine-like
Chaîne SMILES
CCOC(=O)[C@H](O)[C@@H](O)C(=O)OCC
InChI
1S/C8H14O6/c1-3-13-7(11)5(9)6(10)8(12)14-4-2/h5-6,9-10H,3-4H2,1-2H3/t5-,6-/m1/s1
Clé InChI
YSAVZVORKRDODB-PHDIDXHHSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- Synthesis of l-threitol-based crown ethers and their application as enantioselective phase transfer catalyst in Michael additions.: This study synthesizes l-threitol-based crown ethers using diethyl ʟ-tartrate and explores their efficacy as enantioselective phase transfer catalysts in Michael additions, highlighting their potential in asymmetric synthesis (Rapi et al., 2017).
- A facile approach for the synthesis of C13-C24 fragments of maltepolides A, C and D.: This research demonstrates a novel synthesis method for C13-C24 fragments of maltepolides A, C, and D using diethyl ʟ-tartrate, facilitating the study and development of these bioactive compounds (Rao & Srihari, 2016).
- Development of diacyltetrol lipids as activators for the C1 domain of protein kinase C.: This research introduces diacyltetrol lipids synthesized from diethyl ʟ-tartrate, which act as activators for the C1 domain of protein kinase C, offering insights into signal transduction and therapeutic applications (Mamidi et al., 2012).
- Total synthesis of broussonetine F: the orthoamide Overman rearrangement of an allylic diol.: The paper presents the total synthesis of broussonetine F, utilizing diethyl ʟ-tartrate in an orthoamide Overman rearrangement, showcasing a novel synthetic route for complex natural products (Hama et al., 2011).
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
199.4 °F - closed cup
Point d'éclair (°C)
93 °C - closed cup
Équipement de protection individuelle
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique