All Photos(1)
About This Item
Empirical Formula (Hill Notation):
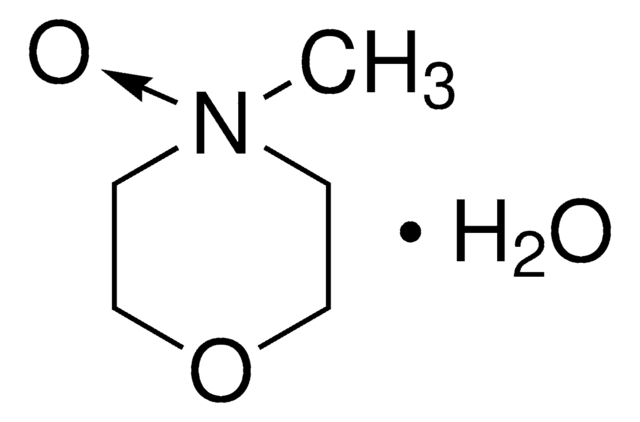
C6H7NO2 · xH2O
CAS Number:
Molecular Weight:
125.13 (anhydrous basis)
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
82-85 °C (lit.)
SMILES string
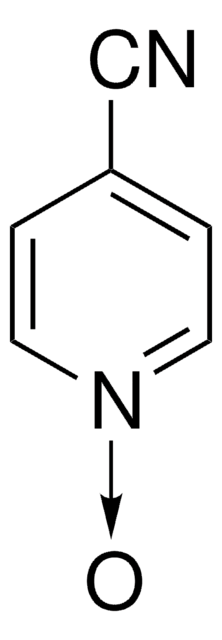
[H]O[H].COc1cc[n+]([O-])cc1
InChI
1S/C6H7NO2.H2O/c1-9-6-2-4-7(8)5-3-6;/h2-5H,1H3;1H2
InChI key
ABJMARLKAXBQBC-UHFFFAOYSA-N
General description
4-Methoxypyridine N-oxide hydrate is a substituted pyridine N-oxide. It forms 1:1 complexes with MnCl2 and NiCl2 salts. Mechanism of reaction between 4-methoxypyridine N-oxide hydrate and bis(pinacolato) (diboron reagent) in CD3CN has been studied by NMR. It forms 1:1 complex with 2,4-dinitrophenol due to the formation of intermolecular hydrogen bond between the O-H and N-O groups.
Application
4-Methoxypyridine N-oxide hydrate may be used in chemical synthesis.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hari Prasad Kokatla et al.
The Journal of organic chemistry, 76(19), 7842-7848 (2011-08-05)
Facile reduction of alkylamino-, anilino-, and pyridyl-N-oxides can be achieved via the use of diboron reagents, predominantly bis(pinacolato)- and in some cases bis(catecholato)diboron [(pinB)(2) and (catB)(2), respectively]. Reductions occur upon simply mixing the amine N-oxide and the diboron reagent in
Binuclear chlorine-bridged complexes of manganese (II) and nickel (II) chlorides with pyridine N-oxides.
Karayannis NM, et al.
Inorganic Chemistry, 8(12), 2559-2562 (1969)
1: 1 Complex of 2, 4-dinitrophenol and 4-methoxypyridine N-oxide hydrate.
Moreno-Fuquen R, et al.
Acta Crystallographica Section E, Structure Reports Online, 57(8), o712-o714 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service