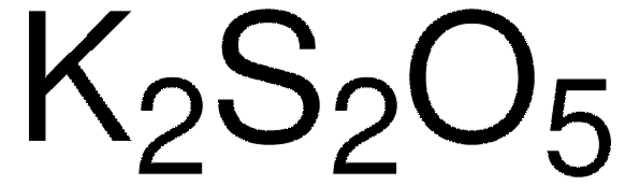P2522
Potassium disulfite
≥98%
Synonym(s):
Potassium metabisulfite, Potassium pyrosulfite
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
K2S2O5
CAS Number:
Molecular Weight:
222.32
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.55
Recommended Products
Assay
≥98%
form
powder
SMILES string
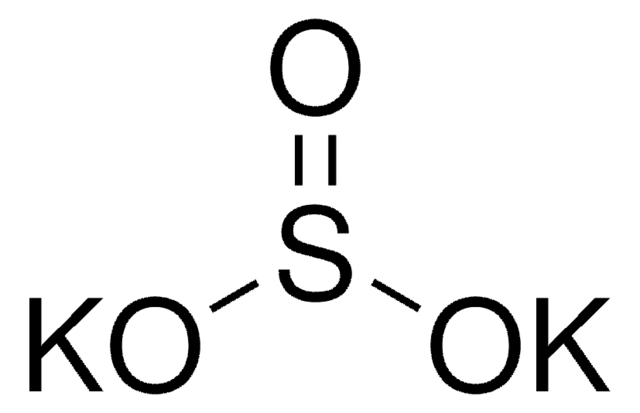
[K+].[K+].[O-]S(=O)S([O-])(=O)=O
InChI
1S/2K.H2O5S2/c;;1-6(2)7(3,4)5/h;;(H,1,2)(H,3,4,5)/q2*+1;/p-2
InChI key
RWPGFSMJFRPDDP-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Potassium disulfite (Potassium metabisulfite, PMB) is an inorganic salt with antimicrobial properties. It is a sulfiting agent that prevents browning of foods. Its genotoxic and cytotoxic effect has been assessed. PMB undergoes hydrolysis to form potassium bisulfite.
Application
Potassium disulfite has been used in a protocol for the modification of the polydimethylsiloxane (PDMS) polymer surfaces.
It may be used in the following processes:
It may be used in the following processes:
- Chemical etching of poly(vinylidene fluoride) in β-phase (β-PVDF) irradiated films.
- Decolorization during the synthesis of 2-iodopyrimidine derivatives.
- Palladium-catalyzed aminosulfonylation of aryl halides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Supplementary Hazards
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Biofunctionalization and self-interaction chromatography in PDMS microchannels.
Deshpande KS, et al.
Biochemical Engineering Journal, 67, 111-119 (2012)
A palladium-catalyzed reaction of aryl halides, potassium metabisulfite, and hydrazines.
Ye S and Wu J.
Chemical Communications (Cambridge, England), 48(80), 10037-10039 (2012)
Amperometric quantification of sodium metabisulfite in pharmaceutical formulations utilizing tetraruthenated porphyrin film modified electrodes and batch injection analysis.
Quintino MSM, et al.
Talanta, 68(4), 1281-1286 (2006)
Nanoporous β-PVDF membranes with selectively functionalized pores.
Cuscito O, et al.
Nucl. Instrum. Methods Phys. Res. Sect. B, 265(1), 309-313 (2007)
Gábor Vlád et al.
The Journal of organic chemistry, 67(18), 6550-6552 (2002-08-31)
A high-yield synthesis was developed for the preparation of 2,2'-bipyrimidine (1) using the Ullmann coupling of 2-iodopyrimidine. The new procedure was also used for the preparation of 4,4',6,6'-tetramethyl-2,2'-bipyrimidine (2) and 5,5'-dibromo-2,2'-bipyrimidine (3).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service