All Photos(1)
About This Item
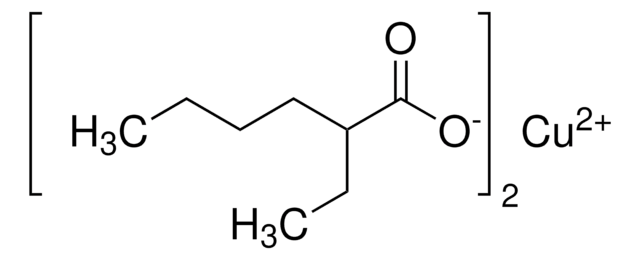
Linear Formula:
[C6H11(CH2)3CO2]2Cu
CAS Number:
Molecular Weight:
402.03
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
form
powder
technique(s)
AAS: suitable
liquid chromatography (LC): suitable
mp
126 °C (dec.) (lit.)
application(s)
battery manufacturing
SMILES string
O=C(CCCC1CCCCC1)O[Cu]OC(=O)CCCC2CCCCC2
InChI
1S/2C10H18O2.Cu/c2*11-10(12)8-4-7-9-5-2-1-3-6-9;/h2*9H,1-8H2,(H,11,12);/q;;+2/p-2
InChI key
FGUOYHAMMRMUQO-UHFFFAOYSA-L
Application
Solutions of copper(II) cyclohexanebutyrate can be used as calibration standards in the flame atomic absorption spectroscopy of copper foils from Li-ion batteries. The salt was used in a study during which copper speciation in jet fuel was determined by liquid chromatography.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Liquid chromatographic determination of copper speciation in jet fuel resulting from dissolved copper
Taylor DB and Synovec RE
Journal of Chromatography A, 659(1), 133-141 (1994)
Quantitation of the dissolution of battery-grade copper foils in lithium-ion battery electrolytes by flame atomic absorption spectroscopy.,
Zhao M, et al.
Electrochimica Acta, 49, 683?689-683?689 (2004)
H J Ougham et al.
Journal of bacteriology, 150(3), 1172-1182 (1982-06-01)
A strain of Arthrobacter was isolated by enrichment culture with cyclohexaneacetate as the sole source of carbon and grew with a doubling time of 4.2 h. In addition to growing with cyclohexaneacetate, the organism also grew with cyclohexanebutyrate at concentrations
Dean M Quesnel et al.
Chemosphere, 84(4), 504-511 (2011-04-05)
Naphthenic acids (NAs) are a major contributor to toxicity in tailings waste generated from bitumen production in the Athabasca Oil Sands region. While investigations have shown that bacteria can biodegrade NAs and reduce tailings toxicity, the potential of algae to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service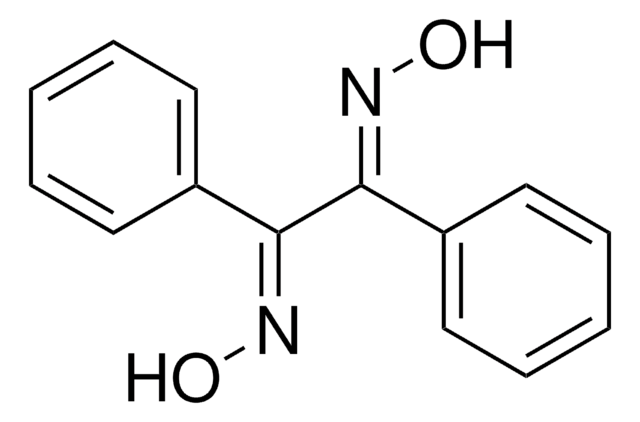




![2-Oxo-7-azaspiro[3.5]nonane-7-carboxylate tert-butyl ester AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/823/725/568e418b-c181-481a-919a-1ff538a6cff0/640/568e418b-c181-481a-919a-1ff538a6cff0.png)


