537011
Prolyl Endopeptidase Inhibitor II
The Prolyl Endopeptidase Inhibitor II, also referenced under CAS 108708-25-4, controls the biological activity of Prolyl Endopeptidase. This small molecule/inhibitor is primarily used for Protease Inhibitors applications.
Synonym(s):
Prolyl Endopeptidase Inhibitor II, Z-PP-CHO
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
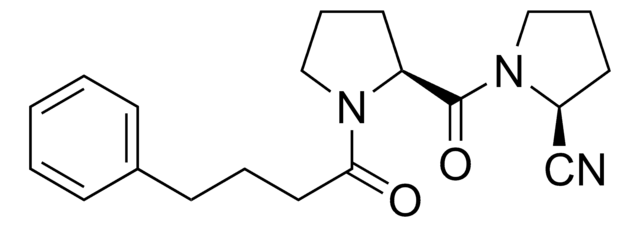
C18H22N2O4
CAS Number:
Molecular Weight:
330.38
UNSPSC Code:
12352200
Recommended Products
Quality Level
Assay
≥97% (HPLC)
form
oil
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
protect from light
color
colorless to off-white
solubility
DMSO: 10 mg/mL
DMF: soluble
ethyl acetate: soluble
shipped in
ambient
storage temp.
−20°C
General description
A cell-permeable dipeptide aldehyde that acts as a specific, potent, slow and tight-binding transition state analog inhibitor of prolyl endopeptidase (Ki = 350 pM and 500 pM for mouse brain and human brain prolyl endopeptidase, respectively). Reported to form a hemiacetal with the active-site serine.
A cell-permeable dipeptide aldehyde that acts as a specific, potent, slow, and tight-binding transition state analog inhibitor of prolyl endopeptidase (Ki = 350 pM and 500 pM for mouse brain and human brain, respectively). Reported to form a hemiacetal with the active site serine of the enzyme.
Biochem/physiol Actions
Cell permeable: yes
Primary Target
Mouse brain prolylendooeotidase
Mouse brain prolylendooeotidase
Product does not compete with ATP.
Reversible: no
Target Ki: 350 pM and 500 pM for mouse brain and human brain prolyl endopeptidase, respectively
Packaging
Packaged under inert gas
Warning
Toxicity: Standard Handling (A)
Sequence
Z-Pro-Pro-CHO
Reconstitution
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
Other Notes
Fülöp, V., et al. 1998. Cell94, 161.
Kahyaoglu, A., et al. 1997. Biochem. J.322, 839.
Bakker, A.V., et al. 1990. Biochem. J.271, 559.
Wilk, S., and Orlowski, M. 1983. J. Neurochem.41, 69.
Kahyaoglu, A., et al. 1997. Biochem. J.322, 839.
Bakker, A.V., et al. 1990. Biochem. J.271, 559.
Wilk, S., and Orlowski, M. 1983. J. Neurochem.41, 69.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Zehavit Goldberg et al.
International journal of molecular sciences, 23(2) (2022-01-22)
The aim of this study was to characterize the distribution of the thrombin receptor, protease activated receptor 1 (PAR1), in the neuroretina. Neuroretina samples of wild-type C57BL/6J and PAR1-/- mice were processed for indirect immunofluorescence and Western blot analysis. Reverse
Valery Golderman et al.
Biomedicines, 10(6) (2022-06-25)
Thrombin is present in peripheral nerves and is involved in the pathogenesis of neuropathy. We evaluated thrombin activity in skin punch biopsies taken from the paws of male mice and rats and from the legs of patients with suspected small-fiber
Chenxi Zhao et al.
Journal of neuroinflammation, 19(1), 189-189 (2022-07-17)
Nafamostat mesylate (nafamostat, NM) is an FDA-approved serine protease inhibitor that exerts anti-neuroinflammation and neuroprotective effects following rat spinal cord injury (SCI). However, clinical translation of nafamostat has been limited by an unclear administration time window and mechanism of action. Time
Mouhannad Malek et al.
Nature communications, 12(1), 2673-2673 (2021-05-13)
Vesicular traffic and membrane contact sites between organelles enable the exchange of proteins, lipids, and metabolites. Recruitment of tethers to contact sites between the endoplasmic reticulum (ER) and the plasma membrane is often triggered by calcium. Here we reveal a
Chenxi Zhao et al.
Neural regeneration research, 19(2), 434-439 (2023-07-25)
Argatroban is a synthetic thrombin inhibitor approved by U.S. Food and Drug Administration for the treatment of thrombosis. However, whether it plays a role in the repair of spinal cord injury is unknown. In this study, we established a rat
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service