All Photos(1)
About This Item
Empirical Formula (Hill Notation):
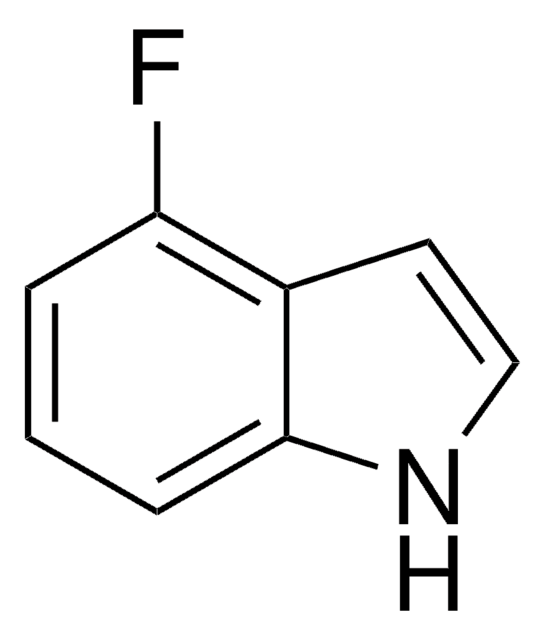
C8H6FN
CAS Number:
Molecular Weight:
135.14
Beilstein:
112192
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
72-76 °C (lit.)
SMILES string
Fc1ccc2cc[nH]c2c1
InChI
1S/C8H6FN/c9-7-2-1-6-3-4-10-8(6)5-7/h1-5,10H
InChI key
YYFFEPUCAKVRJX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
6-Fluoroindole is a halogen substituted indole. Experimental ionization potential of 6-fluoroindole has been evaluated. Preparation of 6-fluoroindole via nitration of indoline has been reported.
Application
6-Fluoroindole may be used as reactant in the preparation of:
- tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- antibacterial agents
- antifungal agents
- Sodium-Dependent Glucose Co-transporter 2 (SGLT2) Inhibitors for the management of hyperglycemia in diabetes
- potent selective serotonin reuptake inhibitors
- inhibitors of HIV-1 attachment
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Na, Y. M.
Bull. Korean Chem. Soc., 31, 3467-3467 (2010)
S Jimmy Budiardjo et al.
ACS synthetic biology, 5(12), 1475-1484 (2016-07-09)
Chemical biology has long sought to build protein switches for use in molecular diagnostics, imaging, and synthetic biology. The overarching challenge for any type of engineered protein switch is the ability to respond in a selective and predictable manner that
David F Cummings et al.
Bioorganic & medicinal chemistry, 18(13), 4783-4792 (2010-06-24)
Efforts to develop ligands that distinguish between clinically relevant 5-HT2A and 5-HT2C serotonin receptor subtypes have been challenging, because their sequences have high homology. Previous studies reported that a novel aplysinopsin belonging to a chemical class of natural products isolated
G S Sheppard et al.
Journal of medicinal chemistry, 37(13), 2011-2032 (1994-06-24)
(2RS,4R)-3-(2-(3-Pyridinyl)thiazolidin-4-oyl)indoles represent a new class of potent, orally active antagonists of platelet activating factor (PAF). The compounds were prepared by acylation of the magnesium or zinc salts of substituted indoles with (2RS,4R)-2-(3-pyridinyl)-3-(tert-butoxycarbonyl)thiazolidin-4-oyl chloride. The 3-acylindole moiety functions as a hydrolytically
Journal of Medicinal Chemistry, 36, 2242-2242 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service