All Photos(1)
About This Item
Linear Formula:
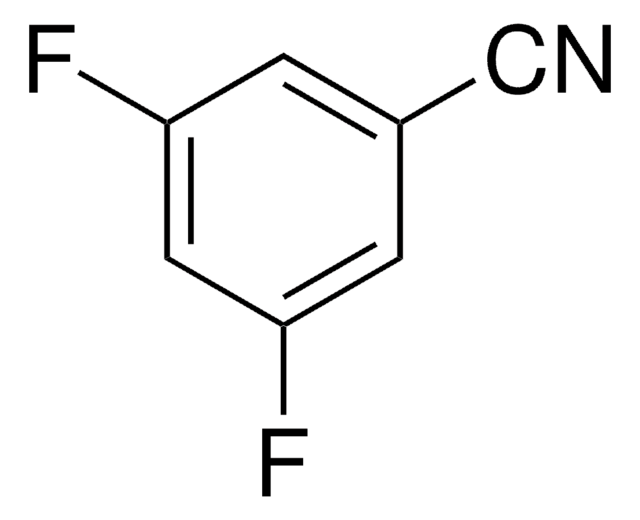
F2C6H3COCH3
CAS Number:
Molecular Weight:
156.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
form:
liquid
Assay:
97%
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.48 (lit.)
bp
76-79 °C/15 mmHg (lit.)
density
1.197 g/mL at 25 °C (lit.)
functional group
fluoro
ketone
SMILES string
CC(=O)c1c(F)cccc1F
InChI
1S/C8H6F2O/c1-5(11)8-6(9)3-2-4-7(8)10/h2-4H,1H3
InChI key
VGIIILXIQLXVLC-UHFFFAOYSA-N
General description
2′,6′-Difluoroacetophenone undergoes ruthenium-catalyzed phenylation with phenylboronate via carbon-fluorine bond cleavage to yield 2′,6′-diphenylacetophenone.
Application
2′,6′-Difluoroacetophenone was used in the synthesis of 2-amino-4-alkyl- and 2-amino-4-arylquinazolines.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
170.6 °F - closed cup
Flash Point(C)
77 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of 2-aminoquinazolines from ortho-fluoroketones.
Hynes JB, et al.
Journal of Heterocyclic Chemistry, 32(4), 1185-1187 (1995)
Ruthenium-catalyzed arylation of fluorinated aromatic ketones via< i> ortho</i>-selective carbon-fluorine bond cleavage.
Kawamoto K, et al.
Tetrahedron Letters, 52(44), 5888-5890 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service