All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H8O3
CAS Number:
Molecular Weight:
188.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
solid
mp
119-122 °C (lit.)
SMILES string
CC(=O)C1=Cc2ccccc2OC1=O
InChI
1S/C11H8O3/c1-7(12)9-6-8-4-2-3-5-10(8)14-11(9)13/h2-6H,1H3
InChI key
CSPIFKKOBWYOEX-UHFFFAOYSA-N
Related Categories
General description
FTIR and FT-Raman spectra of 3-acetylcoumarin has been reported. 3-Acetylcoumarin undergoes condensation with aryl aldehydes in chloroform in the presence of piperidine to yield coumarin derivatives containing 4-arylbut-3-en-2-one moiety.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Abdullah Sulaiman Al-Ayed
Molecules (Basel, Switzerland), 16(12), 10292-10302 (2011-12-14)
A series of new coumarin derivatives 4 containing a 4-arylbut-3-en-2-one moiety were synthesized by condensation of 3-acetylcoumarin 1 with aryl aldehydes 2 in chloroform in the presence of piperidine. The interactions of 3-formyl-4-chlorocoumarin (3) with nitrogen-containg nucleophiles leading to the
X L Huang et al.
Yao xue xue bao = Acta pharmaceutica Sinica, 31(6), 431-436 (1996-01-01)
Twenty-five 3-acetylcoumarin derivatives were synthesized among which twenty-two were not reported before. Antimutagenic activity screen in vitro has shown that some of these compounds have various activities. The structure and activity relationship for 5-, 7-, 8-substituents has been studied. Pharmacological
Wafaa S Hamama et al.
Archiv der Pharmazie, 344(11), 710-718 (2011-09-29)
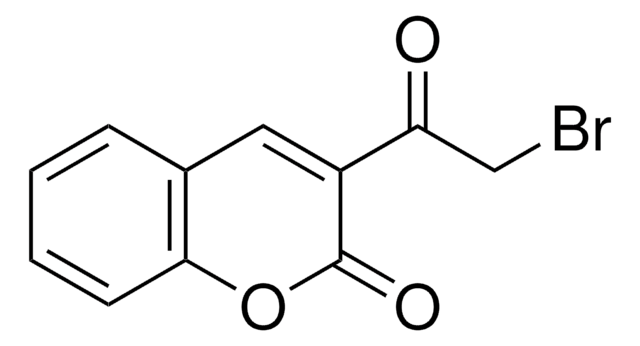
3-Acetylcoumarin (1) was utilized as a key intermediate for the synthesis of 2-aminothiazole derivative 3 via bromination of 1 to afford acetylbromide 2 followed by treatment with thiourea or via Biginelli reaction of 1. Treatment of 3 with 5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-carbaldehyde, 2-methyl-4H-benzo[d][1,3]oxazin-4-one
Jhi Biau Foo et al.
Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine, 31(4), 505-515 (2018-04-07)
Copper complexes have been widely studied for the anti-tumour application as cancer cells are reported to take up greater amounts of copper than normal cells. Preliminary study revealed that the newly synthesised copper complex [Cu(SBCM)2] displayed marked anti-proliferative towards triple-negative
V Arjunan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 109, 79-89 (2013-03-19)
3-Acetylcoumarin (3AC) was synthesised by a Knoevenagel reaction. Conformational analysis using the B3LYP method was also carried out to determine the most stable conformation of the compound. FTIR and FT-Raman spectra of 3AC have been recorded in the range 4000-400
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service