RP-HPLC Analysis of Benzodiazepines on Ascentis® Express Biphenyl
Materialien
Analytische Säule
Produkt-Nr.
Beschreibung
Preisangaben
Standard
Produkt-Nr.
Beschreibung
Preisangaben
Alprazolam -Lösung
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®Clonazepam -Lösung
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®Diazepam -Lösung
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®Flunitrazepam -Lösung
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®Lorazepam -Lösung
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®Nitrazepam -Lösung
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®Temazepam -Lösung
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®CONDITIONS
column
Ascentis Express Biphenyl, 10 cm x 3.0 mm I.D., 2.7 μm particles (64066-U)
mobile phase
[A] Water; [B] acetonitrile; (70:30, A:B)
flow rate
1.0 mL/min
pressure
5294 psi (365 bar)
column temp.
35 °C
detector
UV, 254 nm
injection
1.0 μL
sample
Benzodiazepine Multicomponent Mixture-8 diluted to 50ug/mL in 50:50 Water:Methanol
Beschreibung
Hinweis zur Analyse
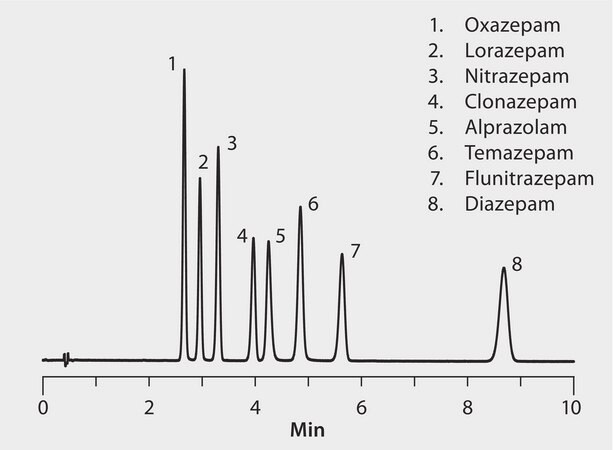
This analysis demonstrates the applicability of the Ascentis Express Biphenyl stationary phase for the analysis of benzodiazepines, a group of psychoactive drugs that act as GABA agonists. The stationary phase provides alternative selectivity and improved resolution to existing phenyl chemistires.
Rechtliche Hinweise
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany