A1632
DL-7-Azatryptophan hydrate
≥98% (TLC)
About This Item
Empfohlene Produkte
product name
DL-7-Azatryptophan hydrate,
Assay
≥98% (TLC)
Form
powder
Farbe
white to off-white
Lagertemp.
−20°C
SMILES String
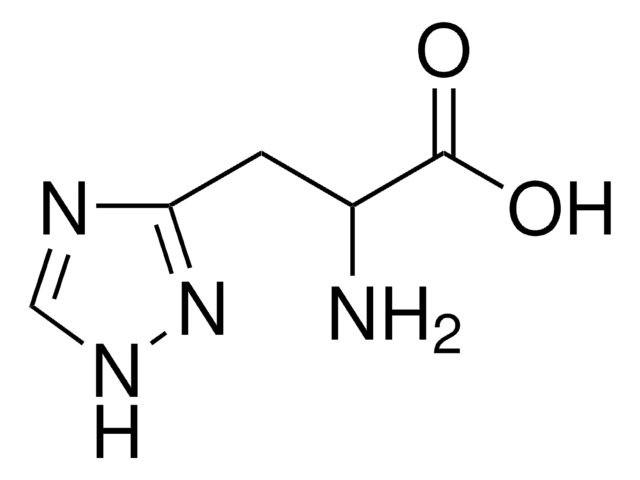
O.NC(Cc1c[nH]c2ncccc12)C(O)=O
InChI
1S/C10H11N3O2.H2O/c11-8(10(14)15)4-6-5-13-9-7(6)2-1-3-12-9;/h1-3,5,8H,4,11H2,(H,12,13)(H,14,15);1H2
InChIKey
PXDRHYQAIUZKHN-UHFFFAOYSA-N
Biochem./physiol. Wirkung
Anwendung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Chromatograms
application for HPLCUnser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.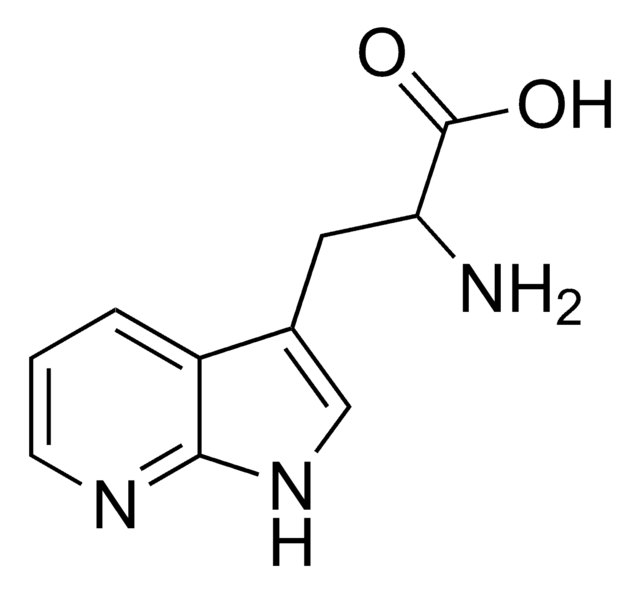



![1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indol 98%](/deepweb/assets/sigmaaldrich/product/structures/181/460/3d58bc34-1b5c-4295-bbac-3b52085670e8/640/3d58bc34-1b5c-4295-bbac-3b52085670e8.png)