145998
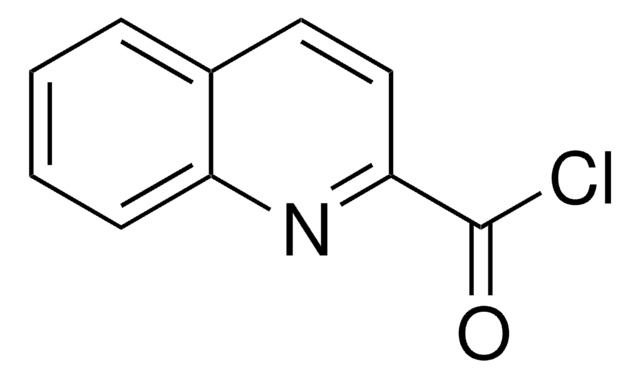
2-Chinoxaloylchlorid
Synonym(e):
2-Chinoxalincarbonylchlorid
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C9H5ClN2O
CAS-Nummer:
Molekulargewicht:
192.60
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
mp (Schmelzpunkt)
113-115 °C (lit.)
Funktionelle Gruppe
acyl chloride
Lagertemp.
2-8°C
SMILES String
ClC(=O)c1cnc2ccccc2n1
InChI
1S/C9H5ClN2O/c10-9(13)8-5-11-6-3-1-2-4-7(6)12-8/h1-5H
InChIKey
SOPDQKNXOCUBSR-UHFFFAOYSA-N
Allgemeine Beschreibung
2-Quinoxaloyl chloride reacts with chiral α-hydroxy carboxylic acids to yield UV and fluorescent derivatives.
Anwendung
2-Quinoxaloyl chloride was used in the synthesis of novel DNA interactive quinoxaline-carbohydrate hybrids possessing disaccharides as the carbohydrate moieties.
Reactant involved in the synthesis of a variety of inhibitors including:
Reactant involved in preparation of PET ligants for breast cancer resistance protein imaging
- Gelatinase inhibitors for cancer treatments
- mGluR5 non-competitve antagonists
- Heterocyclic analogs used as SIRT1 activators
- 3rd Generation multidrug resistance modulators
Reactant involved in preparation of PET ligants for breast cancer resistance protein imaging
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Eye Dam. 1 - Skin Corr. 1B
Lagerklassenschlüssel
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
HPLC resolution of hydroxyl carboxylic acid enantiomers using 2-quinoxaloyl chloride as a new precolumn derivatizing agent.
Brightwell M, et al.
Journal of Liquid Chromatography and Related Technologies, 18(14), 2765-2781 (1995)
Kazunobu Toshima et al.
Bioorganic & medicinal chemistry letters, 14(11), 2777-2779 (2004-05-06)
The novel DNA interactive quinoxaline-carbohydrate hybrids possessing disaccharides as the carbohydrate moieties were designed and synthesized, and their DNA photocleaving abilities were evaluated in order to examine the effect of the disaccharide structures. The configurations of the glycosidic bonds in
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.