8.52072
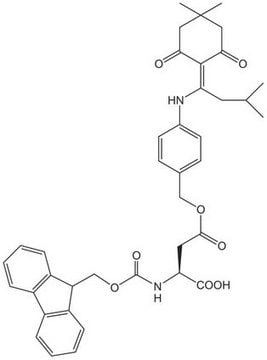
Fmoc-Asp-OAll
Novabiochem®
Synonym(s):
Fmoc-Asp-OAll, N-α-Fmoc-L-aspartic acid α-allyl ester
About This Item
Recommended Products
Quality Level
product line
Novabiochem®
Assay
≥97.0% (acidimetric)
≥98% (TLC)
≥98.0% (HPLC)
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
mp
95 °C (decomposes)
application(s)
peptide synthesis
functional group
carboxylic acid
storage temp.
2-30°C
InChI
1S/C22H21NO6/c1-2-11-28-21(26)19(12-20(24)25)23-22(27)29-13-18-16-9-5-3-7-14(16)15-8-4-6-10-17(15)18/h2-10,18-19H,1,11-13H2,(H,23,27)(H,24,25)/t19-/m0/s1
InChI key
ZJMVIWUCCRKNHY-IBGZPJMESA-N
Related Categories
General description
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] W. Bannwarth, et al. (1992) Tetrahedron Lett., 33, 4557.
[2] F. Albericio, et al. (1993) Tetrahedron Lett., 34, 1549.
[3] J. Eichler, et al. (1994) Pept. Res., 7, 300.
[4] J. Eichler, et al. in ′Peptides 1994, Proc. 23rd European Peptide Symposium′, H. Maia (Eds), ESCOM, Leiden, 1994, pp. 461.
[5] J. Eichler, et al. in ′Solid Phase Synthesis & Combinatorial Libraries, 4th International Symposium′, R. Epton (Eds), Mayflower Scientific Ltd., Birmingham, 1996, pp. 201.
[6] S. A. Kates, et al. in ′Peptides, Chemistry, Structure & Biology, Proc. 13th American Peptide Symposium′, ESCOM, Leiden, 1994, pp. 113.
Linkage
Analysis Note
Appearance of substance (visual): powder
Identity (IR): conforms
Enantiomeric purity: ≥ 99.0 % (a/a)
Purity (TLC(CMA2)): ≥ 98 %
Purity (TLC(157A)): ≥ 98 %
Assay (HPLC, area%): ≥ 98.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Assay (acidimetric): ≥ 97.0 %
Water (K. F.): ≤ 1.5 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Novabiochem® offers orthogonally protected amino acids for peptide synthesis, including cyclic and branched peptides.
Novabiochem® offers orthogonally protected amino acids for peptide synthesis, including cyclic and branched peptides.
Novabiochem® offers orthogonally protected amino acids for peptide synthesis, including cyclic and branched peptides.
Novabiochem® offers orthogonally protected amino acids for peptide synthesis, including cyclic and branched peptides.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service