All Photos(1)
About This Item
Linear Formula:
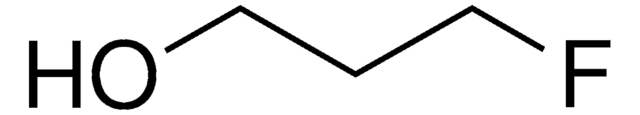
FCH2CH(OH)CH2F
CAS Number:
Molecular Weight:
96.08
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
contains
sodium fluoride as stabilizer
refractive index
n20/D 1.373 (lit.)
bp
54-55 °C/34 mmHg (lit.)
density
1.24 g/mL at 25 °C (lit.)
functional group
fluoro
hydroxyl
SMILES string
OC(CF)CF
InChI
1S/C3H6F2O/c4-1-3(6)2-5/h3,6H,1-2H2
InChI key
PVDLUGWWIOGCNH-UHFFFAOYSA-N
Application
1,3-Difluoro-2-propanol was used in the synthesis of 1,3-difluoroacetone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
107.6 °F - closed cup
Flash Point(C)
42 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M G Feldwick et al.
Journal of biochemical and molecular toxicology, 12(1), 41-52 (1998-01-01)
Administration to rats of 1,3-difluoro-2-propanol (100 mg kg-1 body weight), the major ingredient of the pesticide gliftor, resulted in accumulation of citrate in the kidney after a 3 hour lag phase. 1,3-Difluoro-2-propanol was found to be metabolized to 1,3-difluoroacetone and
K I Menon et al.
Journal of biochemical and molecular toxicology, 15(1), 47-54 (2001-02-15)
The biochemical toxicology of 1,3-difluoroacetone, a known metabolite of the major ingredient of the pesticide Gliftor (1,3-difluoro-2-propanol), was investigated in vivo and in vitro. Rat kidney homogenates supplemented with coenzyme A, ATP, oxaloacetate, and Mg2+ converted 1,3-difluoroacetone to (-)-erythro-fluorocitrate in
M J Garle et al.
Xenobiotica; the fate of foreign compounds in biological systems, 29(5), 533-545 (1999-06-24)
1. This study has examined the ability of dichloropropanols, haloalcohols and their putative metabolites to deplete glutathione when incubated with liver fractions obtained from untreated and differentially induced rats. 2. 1,3-Dichloropropan-2-ol and 2,3-dichloropropan-1-ol (0-1000 microM) both depleted glutathione in a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service