159255
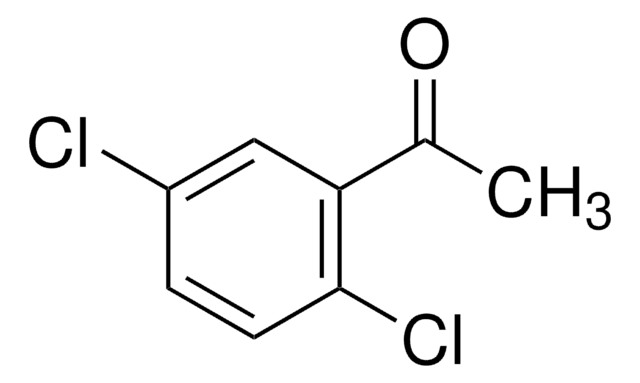
2,2′,4′-Trichloroacetophenone
97%
Synonym(s):
ω,2,4-Trichloroacetophenone, 2,4-Dichlorophenacyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Cl2C6H3COCH2Cl
CAS Number:
Molecular Weight:
223.48
Beilstein:
957098
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
bp
130-135 °C/4 mmHg (lit.)
mp
47-54 °C (lit.)
functional group
chloro
ketone
SMILES string
ClCC(=O)c1ccc(Cl)cc1Cl
InChI
1S/C8H5Cl3O/c9-4-8(12)6-2-1-5(10)3-7(6)11/h1-3H,4H2
InChI key
VYWPPRLJNVHPEU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,2′,4′-Trichloroacetophenone is an α-haloketone. It undergoes reduction to 2′,4′-dichloroacetophenone by glutathione-dependent cytosolic enzymes present in the liver, kidney and brain. It participates in the microwave induced N-alkylation of several azoles to afford the corresponding 1-(2′,4′-dichlorophenacyl)azoles.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Clean and efficient microwave-solvent-free synthesis of 1-(2', 4'-dichlorophenacyl) azoles.
Perez ER, et al.
Tetrahedron, 59(6), 865-870 (2003)
A Brundin et al.
Biochemical pharmacology, 31(23), 3885-3890 (1982-12-01)
alpha-Haloketones are highly reactive compounds, which are known to undergo enzymatic reduction to methyl ketones. The objective of this research was to characterize the enzymes involved in this reaction and to investigate the mechanism of the reaction. 2,2',4'-Trichloroacetophenone was reduced
Justin D Smith et al.
Nature communications, 10(1), 1837-1837 (2019-04-25)
Photocatalytic polymers offer an alternative to prevailing organometallics and nanomaterials, and they may benefit from polymer-mediated catalytic and material enhancements. MPC-1, a polymer photoredox catalyst reported herein, exhibits enhanced catalytic activity arising from charge transfer states (CTSs) between its two
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service