I6125
Yodoacetamida
≥99% (NMR), crystalline
Sinónimos:
α-Iodoacetamide, 2-Iodoacetamide
About This Item
Productos recomendados
biological source
synthetic (organic)
assay
≥99% (NMR)
form
crystalline
mp
92-95 °C (lit.)
solubility
H2O: soluble 0.5 M, clear, colorless
storage temp.
2-8°C
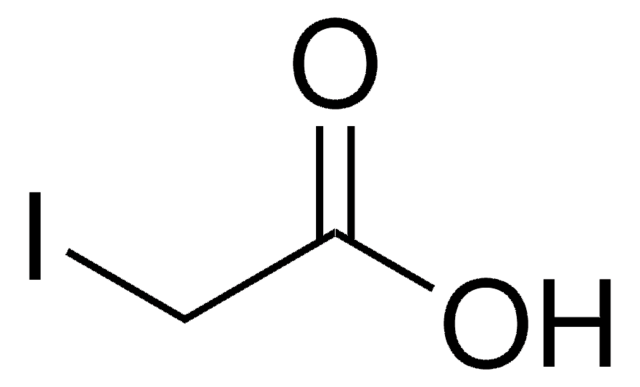
SMILES string
NC(=O)CI
InChI
1S/C2H4INO/c3-1-2(4)5/h1H2,(H2,4,5)
InChI key
PGLTVOMIXTUURA-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- A Mass Spectrometry Strategy for Protein Quantification Based on the Differential Alkylation of Cysteines Using Iodoacetamide and Acrylamide.: This study presents a novel mass spectrometry method for quantifying proteins by differentially alkylating cysteines with iodoacetamide and acrylamide, enhancing the accuracy of protein quantification in complex samples. (Virág et al., 2024).
- Laminarin ameliorates iodoacetamide-induced functional dyspepsia via modulation of 5-HT(3) receptors and the gut microbiota.: Research demonstrates that laminarin can mitigate functional dyspepsia induced by iodoacetamide through modulating 5-HT(3) receptors and altering gut microbiota composition, offering potential therapeutic benefits. (Liu et al., 2024).
- Redox proteomics in melanoma cells: An optimized protocol.: This paper describes an optimized redox proteomics protocol using iodoacetamide, which facilitates the identification of redox-sensitive proteins in melanoma cells, aiding in the understanding of redox regulation in cancer. (Cunha et al., 2024).
- Molecular targets of cisplatin in HeLa cells explored through competitive activity-based protein profiling strategy.: Utilizing iodoacetamide in competitive activity-based protein profiling, this study identifies molecular targets of cisplatin in HeLa cells, providing insights into the drug′s mechanisms. (Chen et al., 2024).
- Development and Comparison of 4-Thiouridine to Cytidine Base Conversion Reaction.: This research compares base conversion reactions involving 4-thiouridine and cytidine, with iodoacetamide playing a crucial role in the reaction mechanism, contributing to advancements in nucleotide chemistry. (Ohashi et al., 2024).
Biochem/physiol Actions
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 4 - Resp. Sens. 1 - Skin Sens. 1
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico