06863
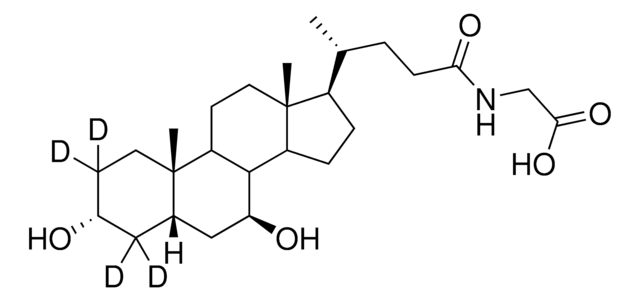
Glycoursodeoxycholic acid
≥96.0% (TLC)
Sinónimos:
N-(3α,7β-Dihydroxy-5β-cholan-24-oyl)glycine, N-[(3α,5β,7β)-3,7-Dihydroxy-24-oxocholan-24-yl]glycine, GUDCA, Glycylursodeoxycholic acid, Ursodeoxycholylglycine
About This Item
Productos recomendados
biological source
synthetic
assay
≥96.0% (TLC)
form
powder
functional group
carboxylic acid
SMILES string
[H][C@@]12[C@]([C@](CC[C@@H](O)C3)(C)[C@]3([H])C[C@@H]2O)([H])CC[C@@]4(C)[C@@]1([H])CC[C@]4([H])[C@]([H])(C)CCC(NCC(O)=O)=O
InChI
1S/C26H43NO5/c1-15(4-7-22(30)27-14-23(31)32)18-5-6-19-24-20(9-11-26(18,19)3)25(2)10-8-17(28)12-16(25)13-21(24)29/h15-21,24,28-29H,4-14H2,1-3H3,(H,27,30)(H,31,32)/t15-,16+,17-,18-,19+,20+,21+,24+,25+,26-/m1/s1
InChI key
GHCZAUBVMUEKKP-XROMFQGDSA-N
Application
- Glycoursodeoxycholic Acid Alleviates Arterial Thrombosis via Suppressing Diacylglycerol Kinases Activity in Platelet.: Highlights the therapeutic potential of Glycoursodeoxycholic acid in alleviating arterial thrombosis by inhibiting diacylglycerol kinase activity in platelets (Yang et al., 2024).
Biochem/physiol Actions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Protocolos
Investigate bile acid roles in gut hormone profiles and glycemic control, vital for clinical labs exploring potential mechanisms.
Contenido relacionado
Bile Acids (BA) are synthesized in the liver and play important roles in cholesterol homeostasis, absorption of vitamins and lipids, and various key metabolic processes.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico