240877
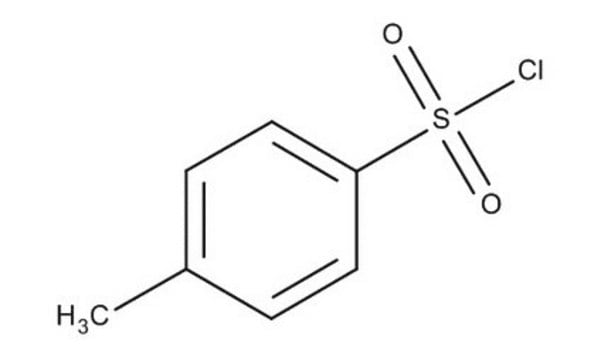
p-Toluenesulfonyl chloride
ReagentPlus®, ≥99%
Sinónimos:
TsCl, Tosyl chloride
About This Item
Productos recomendados
vapor pressure
1 mmHg ( 88 °C)
Quality Level
product line
ReagentPlus®
assay
≥99%
form
solid
bp
134 °C/10 mmHg (lit.)
mp
65-69 °C (lit.)
solubility
benzene: freely soluble(lit.)
chloroform: freely soluble(lit.)
ethanol: freely soluble(lit.)
water: insoluble(lit.)
SMILES string
Cc1ccc(cc1)S(Cl)(=O)=O
InChI
1S/C7H7ClO2S/c1-6-2-4-7(5-3-6)11(8,9)10/h2-5H,1H3
InChI key
YYROPELSRYBVMQ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- In combination with N-methylimidazole for the esterification or thioesterification of carboxylic acids and alcohols or thiols.
- As an additive to enhance the yield of symmetrical biaryls via palladium chloride catalyzed homo-coupling of arylboronic acids in the absence of ligands.
- As a positive chlorine source for the ?-chlorination of ketones.
- Solvent-free tosylation of alcohols and phenols in the presence of heterodoxy acids.
- As an activator for reaction between 2-alkynylbenzaldoxime and phenols to form 1-aroxyisoquinolines in the presence of silver triflate.
- As a catalyst for the solvent-free preparation of symmetrical bis(benzhydryl)ethers from benzhydrols.
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Skin Irrit. 2 - Skin Sens. 1
Storage Class
8B - Non-combustible corrosive hazardous materials
wgk_germany
WGK 1
flash_point_f
262.4 °F - closed cup
flash_point_c
128 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico