616370-M
Aprotinin, Bovine Lung, Crystalline
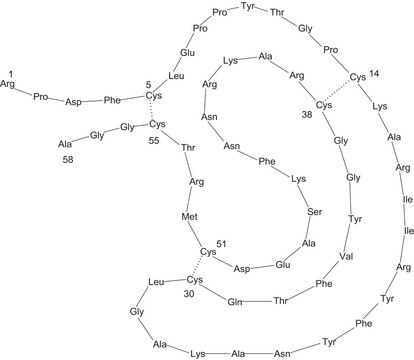
Aprotinin, Bovine Lung, Crystalline, CAS 9087-70-1, is a competitive, reversible, heat- and acid-stable inhibitor of proteolytic and esterolytic activities.
Sinónimos:
Aprotinin, Bovine Lung, Crystalline, Pancreatic Trypsin Inhibitor, Trypsin-Kallikrein Inhibitor
About This Item
Productos recomendados
Quality Level
assay
≥95% (gel filtration chromatography)
form
crystalline solid
specific activity
≥5500 KIU/mg dry wt
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
desiccated (hygroscopic)
impurities
≤10 EU/mg endotoxin
color
white to off-white
pI
10.5
solubility
water: 10 mg/mL
storage temp.
2-8°C
General description
Biochem/physiol Actions
chymotrypsin
Warning
Unit Definition
Reconstitution
Other Notes
Roberts, R.F., et al. 1998. Ann. Thorac. Surg.66, 225.
Azougagh Oualane, F., et al. 1992. Thromb. Res.68, 185.
Anderson, L.O., et al. 1986. Proc. Natl. Acad. Sci. USA83, 2979.
Kruithof, W.L., et al. 1986. Biochem. J.226, 631.
McKeehan, W.L., et al. 1986. J. Biol. Chem.261, 5378.
Fritz, H., and Wunderer, G. 1983. Arzneim. Forsch.33, 479.
Rijken, D.C., and Collen, D. 1981. J. Biol. Chem.256, 7035.
Kassell, B., et al. 1970. Methods Enzymol.19, 845.
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico