755745
2,2′-Azobis(2-methylpropionitrile)
recrystallized from methanol, 99%
Sinónimos:
α,α′-Azoisobutyronitrile, AIBN, Azobisisobutyronitrile, Free radical initiator
About This Item
Productos recomendados
Quality Level
assay
99%
form
crystals
mp
102-104 °C (dec.) (lit.)
103-107 °C
storage temp.
−20°C
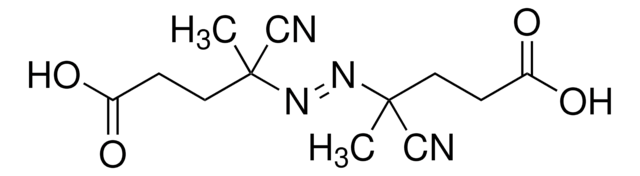
SMILES string
CC(C)(\N=N\C(C)(C)C#N)C#N
InChI
1S/C8H12N4/c1-7(2,5-9)11-12-8(3,4)6-10/h1-4H3/b12-11+
InChI key
OZAIFHULBGXAKX-VAWYXSNFSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- Porous Acid-Base Hybrid Polymers for Enhanced NH3 Uptake: This study discusses the use of 2,2′-Azobis(2-methylpropionitrile) in the synthesis of acid-base hybrid polymers, highlighting its role in enhancing ammonia uptake through cooperative hydrogen bonds (X Luo, Y Liu, et al., 2023).
- Extraction of Fluoroquinolones from Milk: The development of molecularly imprinted polymers using 2,2′-Azobis(2-methylpropionitrile) as an initiator for the extraction of antibiotics from milk showcases its application in food safety and pharmaceutical analysis (E Megias-Pérez, et al., 2023).
- Thermo-responsive Copolymer Visible Light Catalyst: Highlighting the use of 2,2′-Azobis(2-methylpropionitrile) in the synthesis of thermo-responsive copolymers, this study explores its applications in catalysis and material science, particularly in photoreactive polymers (S Wu, et al., 2024).
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Self-react. C
supp_hazards
Storage Class
4.1A - Other explosive hazardous materials
wgk_germany
WGK 2
flash_point_f
122.0 °F
flash_point_c
50 °C
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico