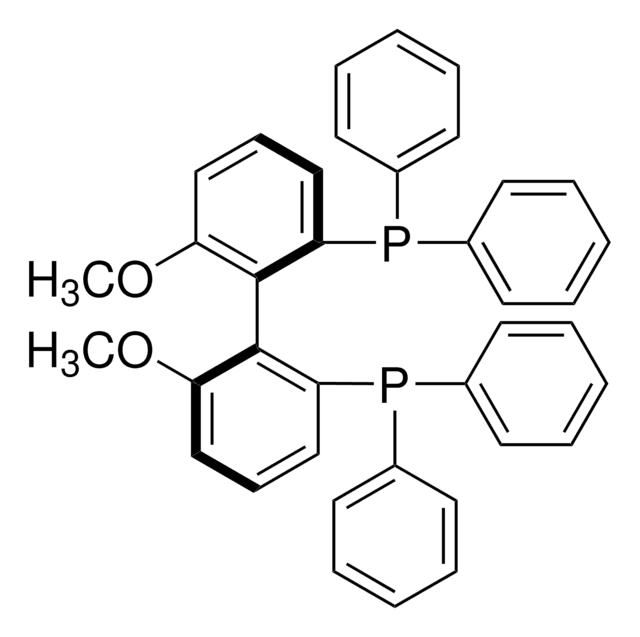
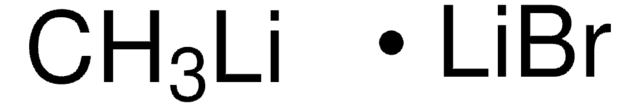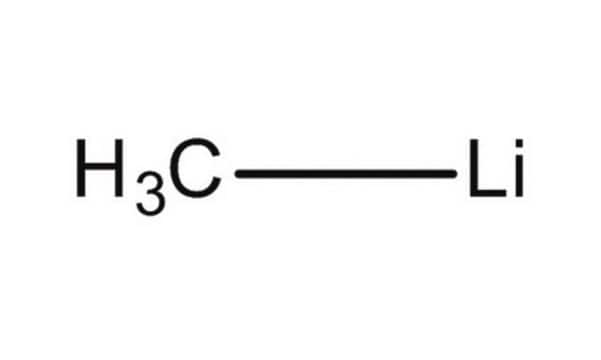693537
R-MOP
≥94%
Sinónimos:
(R)-(+)-2-(Diphenylphosphino)-2′-methoxy-1,1′-binanaphthyl
About This Item
Productos recomendados
assay
≥94%
form
solid
optical activity
[α]20/D +94°, c = 0.5 in chloroform
SMILES string
COc1ccc2ccccc2c1-c3c(ccc4ccccc34)P(c5ccccc5)c6ccccc6
InChI
1S/C33H25OP/c1-34-30-22-20-24-12-8-10-18-28(24)32(30)33-29-19-11-9-13-25(29)21-23-31(33)35(26-14-4-2-5-15-26)27-16-6-3-7-17-27/h2-23H,1H3
InChI key
KRWTWSSMURUMDE-UHFFFAOYSA-N
General description
Application
Ligand used in palladium-catalyzed asymmetric hydrosilylation of olefins, palladium-catalyzed reduction of allylic esters, rhodium-catalyzed asymmetric addition reactions, and asymmetric amination reactions catalyzed by copper(I) complexes.
Legal Information
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Chiral diene ligands enable asymmetric transformations, constructing enantioenriched compounds from achiral substrates efficiently.
Hydrogenation, Asymmetric Catalysis, Binap, SEGPHOS®, Aldol reaction, Alkenylation, Arylation, Mannich reaction, Fluorination, Michael addition, Hydrosilylation, Cycloaddition, Takasago
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-bis(difenilfosfino)ferroceno]dicloropaladio(II), complejo con diclorometano](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
![(S)-(+)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a;3,4- a′]dinaphthalen-4-yl)dimethylamine 97%](/deepweb/assets/sigmaaldrich/product/structures/400/008/628143de-3954-440a-ba9c-4c0ff8e44663/640/628143de-3954-440a-ba9c-4c0ff8e44663.png)
![(R)-(–)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane 96%](/deepweb/assets/sigmaaldrich/product/structures/131/143/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2/640/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2.png)