122408
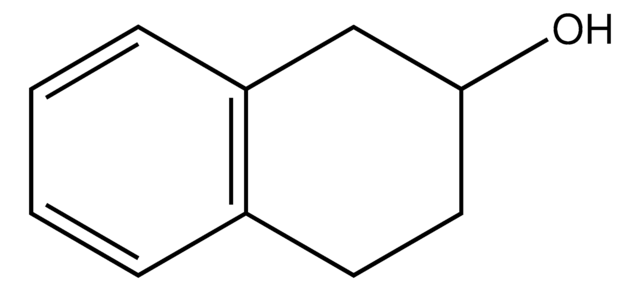
1,2,3,4-Tetrahydro-1-naphthol
97%
Sinónimos:
α-Tetralol
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C10H12O
Número de CAS:
Peso molecular:
148.20
Beilstein/REAXYS Number:
2046227
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
97%
form
solid
refractive index
n20/D 1.564 (lit.)
bp
102-104 °C/2 mmHg (lit.)
density
1.09 g/mL at 25 °C (lit.)
SMILES string
OC1CCCc2ccccc12
InChI
1S/C10H12O/c11-10-7-3-5-8-4-1-2-6-9(8)10/h1-2,4,6,10-11H,3,5,7H2
InChI key
JAAJQSRLGAYGKZ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
(R)-(-)-enantiomer of 1,2,3,4-Tetrahydro-1-naphthol is a substrate for aryl sulfotransferase (AST) IV enzyme and (S)-(+)-1,2,3,4-tetrahydro-1-naphthol is a competitive inhibitor of AST IV-catalyzed sulfation of 1-naphthalenemethanol. It is the major urinary metabolite of tetralin.
Application
1,2,3,4-Tetrahydro-1-naphthol was used as chiral probe to examine the role of three aromatic residues in enzyme-substrate interactions at the sulfuryl acceptor binding site of aryl sulfotransferase IV enzyme.
signalword
Warning
hcodes
pcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Metabolism of tetralin and toxicity of Cuprex in man.
D E Drayer et al.
Drug metabolism and disposition: the biological fate of chemicals, 1(3), 577-579 (1973-05-01)
Jonathan J Sheng et al.
Drug metabolism and disposition: the biological fate of chemicals, 32(5), 559-565 (2004-04-22)
Aryl sulfotransferase (AST) IV (also named tyrosine-ester sulfotransferase and ST1A1) is a major phenol sulfotransferase in the rat, and it catalyzes the sulfation of many drugs, carcinogens, and other xenobiotics that contain phenol, benzylic alcohol, N-hydroxy arylamine, and oxime functional
Vyas Sharma et al.
Journal of medicinal chemistry, 45(25), 5514-5522 (2002-12-03)
Comparative Molecular Field Analysis (CoMFA) methods were used to produce a 3D-QSAR model that correlated the catalytic efficiency of rat hepatic aryl sulfotransferase (AST) IV, expressed as log(k(cat)/K(m)), with the molecular structures of its substrates. A total of 35 substrate
W F Leebaw et al.
The Journal of clinical endocrinology and metabolism, 47(3), 480-487 (1978-09-01)
Although the role of the neurotransmitter, dopamine (DA), in the regulation of PRL has been well documented, controversy exists regarding its participation in the regulation of the other pituitary hormones. Consequently, we infused DA into six healthy male subjects (ages
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)