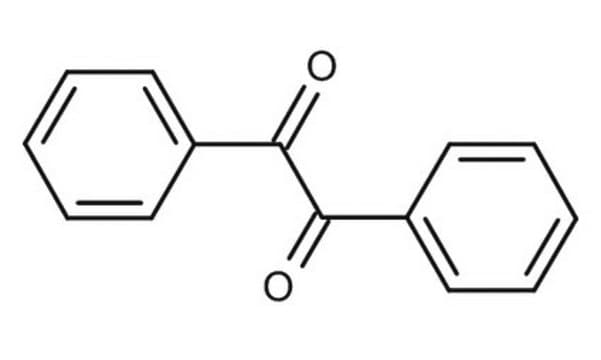
11970
1,3-Diphenyl-2-propenone
≥98.0% (GC)
Sinónimos:
Benzalacetophenone, Chalcone
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
C6H5CH=CHCOC6H5
Número de CAS:
Peso molecular:
208.26
Beilstein:
1210466
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Ensayo
≥98.0% (GC)
solubilidad
dioxane: soluble 1 g/10 mL, clear, colorless
grupo funcional
ketone
phenyl
cadena SMILES
O=C(\C=C\c1ccccc1)c2ccccc2
InChI
1S/C15H12O/c16-15(14-9-5-2-6-10-14)12-11-13-7-3-1-4-8-13/h1-12H/b12-11+
Clave InChI
DQFBYFPFKXHELB-VAWYXSNFSA-N
Información sobre el gen
rat ... Ar(24208)
Aplicación
1,3-Diphenyl-2-propenone was used in the preparation of pharmacologically-interesting heterocyclic systems like pyrazolines and pyrimidines.
Acciones bioquímicas o fisiológicas
1,3-Diphenyl-2-propenone (chalcone) inhibits the proliferation of human breast cancer cell lines, MCF-7 and MDA-MB-231 by inducing apoptosis and blocking cell cycle progression in the G2/M phase. It is an inhibitor of Plasmodium falciparum cyclin-dependent protein kinases.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Lijuan Song et al.
Fitoterapia, 84, 107-114 (2012-11-20)
Hydroxysafflor yellow A (HSYA) is an active ingredient obtained from the flower of Carthamus tinctorius L. The present study investigated the effects of HSYA on lipopolysaccharide (LPS)-induced inflammatory signal transduction in human alveolar epithelial A549 cells. A549 cells stimulated with
Yau-Hung Chen et al.
Molecules (Basel, Switzerland), 18(2), 2052-2060 (2013-02-07)
The aim of this study was to investigate novel chalcones with potent anti-inflammatory activities in vivo. Chalcone and two chalcone analogues (compound 5 and 9) were evaluated using a caudal fin-wounded transgenic zebrafish line "Tg(mpx:gfp)" to visualize the effect of
Zijing Li et al.
Journal of medicinal chemistry, 56(2), 471-482 (2012-12-18)
Rhenium and technetium-99m cyclopentadienyl tricarbonyl complexes mimicking the chalcone structure were prepared. These complexes were proved to have affinity to β-amyloid (Aβ) in fluorescent staining on brain sections of Alzheimer's Disease (AD) patient and binding assay using Aβ(1-42) aggregates, with
Conventional and Microwave assisted Synthesis of 1, 3- Diphenyl -2- Propenone derivatives and Cytotoxic, Anti bacterial activites.
Ahmad MR and Bano N.
International Journal of ChemTech Research, 3(3), 1470-1478 (2011)
Selective inhibition of Pfmrk, a Plasmodium falciparum CDK, by antimalarial 1,3-diaryl-2-propenones.
Jeanne A Geyer et al.
Bioorganic & medicinal chemistry letters, 19(7), 1982-1985 (2009-03-03)
The cyclin dependent protein kinases, Pfmrk and PfPK5, most likely play an essential role in cell cycle control and differentiation in Plasmodium falciparum and are thus an attractive target for antimalarial drug development. Various 1,3-diaryl-2-propenones (chalcone derivatives) which selectivity inhibit
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico