109606
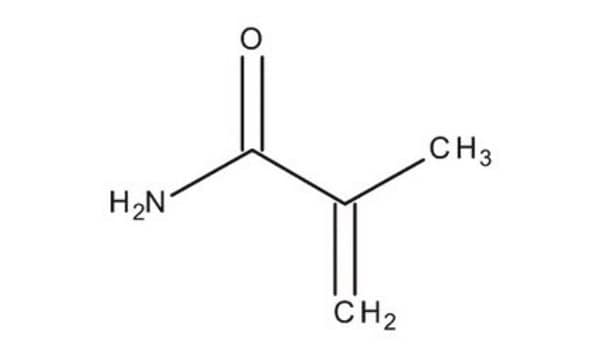
Methacrylamide
98%
Sinónimos:
2-Methylacrylamide, 2-Methylpropenamide, Methacrylic acid amide
About This Item
Productos recomendados
assay
98%
form
solid
mp
106-110 °C (lit.)
SMILES string
CC(=C)C(N)=O
InChI
1S/C4H7NO/c1-3(2)4(5)6/h1H2,2H3,(H2,5,6)
InChI key
FQPSGWSUVKBHSU-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- Synthesis of non-fouling poly brushes by photoinduced SET-LRP: This study highlights the use of photoinduced SET-LRP for the polymerization of methacrylamide, emphasizing its efficiency and the quality of the resulting polymers for non-fouling applications (M Vorobii, A de los Santos Pereira, 2015).
- Hydrolytic stability of methacrylamide and methacrylate in gelatin methacryloyl: The study investigates the hydrolytic stability of methacrylamide within gelatin methacryloyl, highlighting its stability and potential in biomedical applications (J Zheng, M Zhu, G Ferracci, NJ Cho, 2018).
- Two-step mechanisms of tumor selective delivery of N-(2-hydroxypropyl) methacrylamide copolymer conjugated with pirarubicin via an acid-cleavable linkage: This paper discusses the development of a copolymer conjugate for targeted cancer therapy, showcasing a two-step mechanism for enhanced drug delivery (H Nakamura, T Etrych, P Chytil, M Ohkubo, 2014).
- Backbone Degradable N-(2-Hydroxypropyl)methacrylamide Copolymer Conjugates with Gemcitabine and Paclitaxel: The research focuses on degradable copolymer conjugates for delivering cancer therapeutics, noting significant effects on tumor reduction and highlighting the impact of molecular weight (J Yang, R Zhang, H Pan, Y Li, Y Fang, 2017).
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - STOT RE 2 - STOT SE 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico