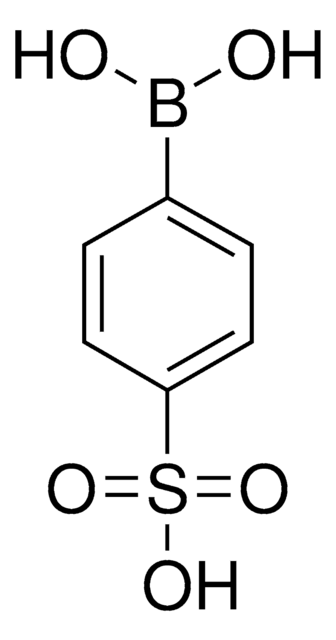
675903
4-(Methanesulfonyl)phenylboronic acid
≥95.0%
Synonym(s):
4-(Methanesulfonyl)benzeneboronic acid, 4-(Methylsulfonyl)phenylboronic acid, 4-Methansulfonylphenylboronic acid
About This Item
Recommended Products
Assay
≥95.0%
form
solid
mp
289-293 °C
SMILES string
CS(=O)(=O)c1ccc(cc1)B(O)O
InChI
1S/C7H9BO4S/c1-13(11,12)7-4-2-6(3-5-7)8(9)10/h2-5,9-10H,1H3
InChI key
VDUKDQTYMWUSAC-UHFFFAOYSA-N
Related Categories
General description
Application
- sequential Suzuki cross-coupling reactions
- Copper-catalyzed oxidative trifluoromethylthiolation of aryl boronic acids
- directed metalation and regioselective functionalization of 3-bromofuran and related heterocycles
- Barton-Zard pyrrole cyclocondensations and Baeyer-Villiger oxidations
- diplar cycloaddition and palladium-catalyzed cross-coupling processes
- continuous flow Suzuki reactions for odanacatib intermediate synthesis
Reagent used in Preparation of
- diarylaminopyridines as potential anti-malarial agents
- hydropyranopyrazine via chloropyrazinecarboxaldehyde and olefination
- biaryl sulfone derivatives as antagonists of the histamine H3 receptor
- novel kinase inhibitor scaffolds with potential antitumor effects
- Hepatitis C virus inhibition activity of N-hydroxyisoquinoline di
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service