All Photos(1)
About This Item
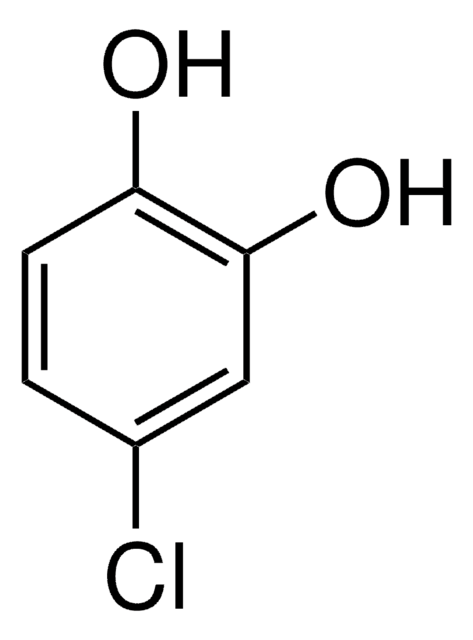
Linear Formula:
Cl2C6H2(OH)2
CAS Number:
Molecular Weight:
179.00
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
98%
mp
168-171 °C (lit.)
SMILES string
Oc1cc(Cl)c(O)cc1Cl
InChI
1S/C6H4Cl2O2/c7-3-1-5(9)4(8)2-6(3)10/h1-2,9-10H
InChI key
AYNPIRVEWMUJDE-UHFFFAOYSA-N
General description
2,5-Dichlorohydroquinone (2,5-DCHQ) is a hydroquinone derivative that can be synthesized by reducing 2,5-dichloroquinone using sodium dithionite (Na2S2O4). The kinetics of the reaction of 2,5-DCHQ and N-phenyl-1,4-benzoquinonemonoimine has been studied. The transformation of 2,5-DCHQ to 2-chloromaleylacetate using PcpA (Pcp = pentachlorophenol) protein, isolated from Escherichia coli has been investigated. It is reported to be the degradation product of 2,4,5-trichlorophenoxyacetic acid and γ-hexachlorocyclohexane.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
K Miyauchi et al.
Journal of bacteriology, 180(6), 1354-1359 (1998-03-27)
Sphingomonas (formerly Pseudomonas) paucimobilis UT26 utilizes gamma-hexachlorocyclohexane (gamma-HCH), a halogenated organic insecticide, as a sole carbon and energy source. In a previous study, we showed that gamma-HCH is degraded to 2,5-dichlorohydroquinone (2,5-DCHQ) (Y. Nagata, R. Ohtomo, K. Miyauchi, M. Fukuda
S Oikawa et al.
Carcinogenesis, 17(12), 2733-2739 (1996-12-01)
p-Dichlorobenzene (p-DCB) has been reported to be carcinogenic for rodents, although it does not seem to be mutagenic in bacterial test systems. In this study, the mechanism of DNA damage by metabolites of p-DCB in the presence of metals was
Y Nagata et al.
Journal of bacteriology, 176(11), 3117-3125 (1994-06-01)
In Pseudomonas paucimobilis UT26, gamma-hexachlorocyclohexane (gamma-HCH) is converted to 2,5-dichloro-2,5-cyclohexadiene-1,4-diol (2,5-DDOL), which is then metabolized to 2,5-dichlorohydroquinone. Here, we isolated from the genomic library of UT26 two genes which expressed 2,5-DDOL dehydrogenase activity when they were transformed into P. putida
R A Haugland et al.
Applied and environmental microbiology, 56(5), 1357-1362 (1990-05-01)
Combined cell suspensions of the 2,4,5-trichlorophenoxyacetic acid (2,4,5-T)-metabolizing organism Pseudomonas cepacia AC1100, and the 2,4-dichlorophenoxyacetic acid (2,4-D)-metabolizing organism Alcaligenes eutrophus JMP134 were shown to effectively degrade either of these compounds provided as single substrates. These combined cell suspensions, however, poorly
Y Ohtsubo et al.
FEBS letters, 459(3), 395-398 (1999-10-20)
The pentachlorophenol (PCP) mineralizing bacterium Sphingomonas chlorophenolica ATCC39723 degrades PCP via 2,6-dichlorohydroquinone (2,6-DCHQ). The pathway converting PCP to 2,6-DCHQ has been established previously; however, the pathway beyond 2,6-DCHQ is not clear, although it has been suggested that a PcpA plays
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service