All Photos(1)
About This Item
Linear Formula:
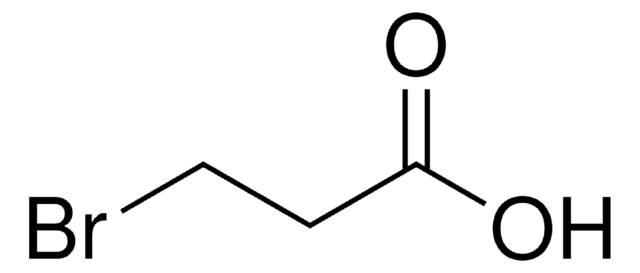
CH3CHBrCH2COOH
CAS Number:
Molecular Weight:
167.00
Beilstein:
1720574
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0% (GC)
refractive index
n20/D 1.476
density
1.57 g/mL at 20 °C (lit.)
SMILES string
CC(Br)CC(O)=O
InChI
1S/C4H7BrO2/c1-3(5)2-4(6)7/h3H,2H2,1H3,(H,6,7)
InChI key
HAIUIAZIUDPZIE-UHFFFAOYSA-N
Application
3-Bromobutyric acid was used in the synthesis of β-butyrolactone, monomer for a naturally occurring polyester, D-poly-β-hydroxybutyrate. It was used to investigate the effect of halo acids on the activity of aspartate aminotransferase isoenzymes in their pyridoxamine form. It was also used in the synthesis of optically active β-lactones.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
237.2 °F - closed cup
Flash Point(C)
114 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and characterization of poly-?-hydroxybutyrate. I. Synthesis of crystalline DL-poly-?-hydroxybutyrate from DL-?-butyrolactone.
Agostini DE, et al.
Journal of Polymer Science: Part A, General Papers, 9(10), 2775-2787 (1971)
Methods for the synthesis of optically active β-lactones (2-oxetanones).
Yang YW and Romo D.
Tetrahedron, 55(21), 6403-6434 (1999)
Selective inactivation of pyridoxamine form of aspartate aminotransferase by iodoacetate. Carboxymethylation of 4'-amino group of bound pyridoxamine 5'-phosphate.
Y Morino et al.
The Journal of biological chemistry, 253(17), 6026-6030 (1978-09-10)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![2-[2-(2-Aminoethoxy)ethoxy]ethanol ≥96.0% (GC)](/deepweb/assets/sigmaaldrich/product/structures/237/185/b94eadd2-5a2c-4a5b-a13d-3d3905df0dbc/640/b94eadd2-5a2c-4a5b-a13d-3d3905df0dbc.png)