All Photos(1)
About This Item
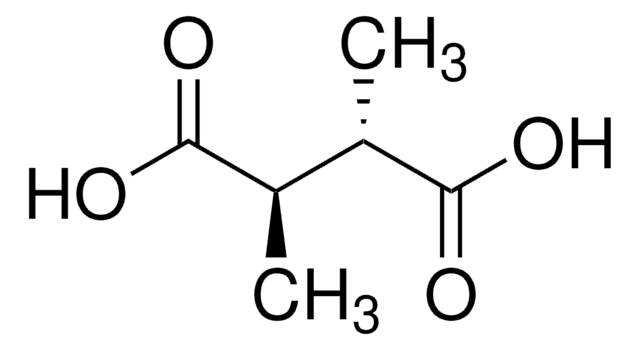
Linear Formula:
HOOCCH(CH3)CH(CH3)COOH
CAS Number:
Molecular Weight:
146.14
Beilstein:
1723932
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
powder
SMILES string
CC(C(C)C(O)=O)C(O)=O
InChI
1S/C6H10O4/c1-3(5(7)8)4(2)6(9)10/h3-4H,1-2H3,(H,7,8)(H,9,10)
InChI key
KLZYRCVPDWTZLH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
P S Thomas et al.
Postgraduate medical journal, 67(783), 63-65 (1991-01-01)
Chronic lead poisoning has traditionally been treated by parenteral agents. We present a case where a comparison of ethylene diaminetetra-acetic acid was made with 2,3-dimethyl succinic acid (DMSA) which has the advantage of oral administration associated with little toxicity and
G Branlant
European journal of biochemistry, 121(2), 407-411 (1982-01-01)
Aldehyde reductase I from pig liver is strongly inhibited by cyclized NADP--2-oxodiacid adducts. This result, in conjunction with those showing a strong inhibitory effect of certain diacid derivatives, such as (+/-)-dimethylsuccinic acid, towards aldehyde reductase I, suggests the presence of
E Asante-Appiah et al.
Biochemistry, 36(29), 8710-8715 (1997-07-22)
gem-Dimethylsuccinic acid and its higher homolog, 2-methyl-2-ethylsuccinic acid (MESA) are highly potent inhibitors of both carboxypeptidase A (CPA) and B. The inhibition constant of MESA for CPA (0.11 microM for the racemic mixture) is remarkable considering the relatively simple structure
Birgitta C Burckhardt et al.
Journal of the American Society of Nephrology : JASN, 13(11), 2628-2638 (2002-10-25)
The active transport of Krebs cycle intermediates, such as succinate, alpha-ketoglutarate, and citrate, is mediated by sodium-coupled transporters found in the luminal (NaDC-1) and basolateral plasma membranes (NaDC-3) of proximal tubule cells. This study used the two-electrode voltage clamp technique
Jannick Jacobsen et al.
Dalton transactions (Cambridge, England : 2003), 48(23), 8433-8441 (2019-05-23)
Six different chiral and achiral alkane dicarboxylic C4-acids, i.e. succinic acid (H2SUC), dl-2-methylsuccinic acid (H2MS), 2,3-dimethylsuccinic acid (H2DMS) and aspartic acid (d-, l- and dl-H2ASP), were used to obtain Ce(iv)-MOFs via microwave assisted reactions. In water-based syntheses, MOFs with three
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service