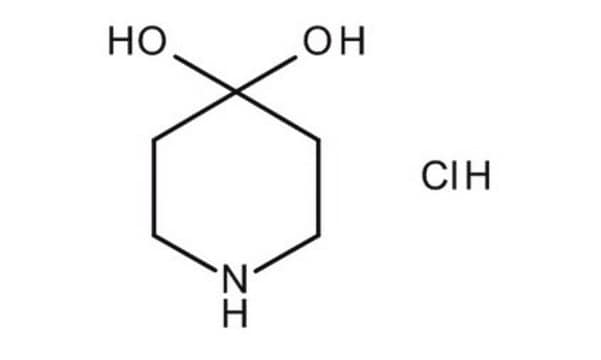
B29806
1-Benzyl-4-piperidone
99%
Synonym(s):
1-Benzyl-4-oxopiperidine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H15NO
CAS Number:
Molecular Weight:
189.25
Beilstein:
128556
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.541 (lit.)
bp
134 °C/7 mmHg (lit.)
density
1.021 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
O=C1CCN(CC1)Cc2ccccc2
InChI
1S/C12H15NO/c14-12-6-8-13(9-7-12)10-11-4-2-1-3-5-11/h1-5H,6-10H2
InChI key
SJZKULRDWHPHGG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-Benzyl-4-piperidone serves as a versatile heterocyclic building block in the synthesis of pharmaceutical compounds.
Application
1-Benzyl-4-piperidone is generally used as a building block in the synthesis of various medicinal compounds. Applications include the synthesis of:
- Spirocyclic furopyridines as haloperidol-sensitive σ receptor ligands.
- Multi-target-directed donepezil + propargylamine + 8-hydroxyquinoline (DPH) hybrids for the treatment of Alzheimer′s disease.
- Spiropiperidine iminohydantoin aspartyl protease inhibitors.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
159.8 °F
Flash Point(C)
71 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Donepezil+ propargylamine+ 8-hydroxyquinoline hybrids as new multifunctional metal-chelators, ChE and MAO inhibitors for the potential treatment of Alzheimer's disease.
Wang Li, et al.
European Journal of Medicinal Chemistry, 80(2), 543-561 (2014)
Novel spiropiperidines as highly potent and subtype selective ?-receptor ligands. Part 1.
Maier CA and Wunsch B
Journal of Medicinal Chemistry, 45(2), 438-448 (2002)
Synthesis and ? receptor affinity of regioisomeric spirocyclic furopyridines.
Miyata K, et al.
European Journal of Medicinal Chemistry, 83(2), 709-716 (2014)
Di-tert-butylsilylene-directed α-selective synthesis of 4-methylumbelliferyl T-antigen.
Imamura A, et al.
Organic Letters, 7(20), 4415-4418 (2005)
Chunmei Gao et al.
Bioorganic & medicinal chemistry, 16(18), 8670-8675 (2008-08-21)
A novel series of 10-benzyl-9(10H)-acridinones and 1-benzyl-4-piperidones were synthesized and tested for their in vitro antitumor activities against CCRF-CEM cells. Assay-based antiproliferative activity study using CCRF-CEM cell lines revealed that the acridone group and the substitution pattern on the benzene
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service