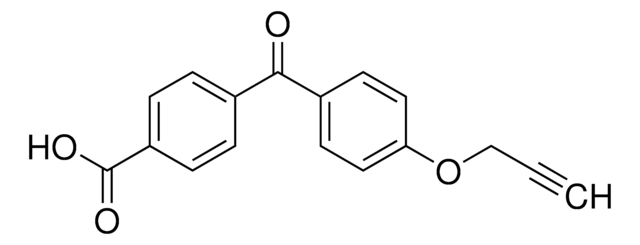
B12407
4-Benzoylbenzoic acid
99%
Synonym(s):
p-Benzoylbenzoic acid, Benzophenone-4-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5COC6H4CO2H
CAS Number:
Molecular Weight:
226.23
Beilstein:
1960224
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
crystals
mp
198-200 °C (lit.)
SMILES string
OC(=O)c1ccc(cc1)C(=O)c2ccccc2
InChI
1S/C14H10O3/c15-13(10-4-2-1-3-5-10)11-6-8-12(9-7-11)14(16)17/h1-9H,(H,16,17)
InChI key
IFQUPKAISSPFTE-UHFFFAOYSA-N
Gene Information
human ... ACHE(43) , BCHE(590) , CES1(1066)
Looking for similar products? Visit Product Comparison Guide
General description
4-Benzoylbenzoic acid is a benzophenone derivative. It can undergo hydrogenolysis to 4-benzylbenzoic acid. Cotton fabrics incorporated with 4-benzoylbenzoic acid have shown pesticide degradation ability, when exposed to UV irradiation.
Application
4-Benzoylbenzoic acid, along with methacrylic acid can be used as ligands for synthesizing a novel Tb(III) ternary complex with luminescent property.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Antimicrobial and chemical detoxifying functions of cotton fabrics containing different benzophenone derivatives.
Hong K
Carbohydrate Polymers, 71(4), 598-605 (2008)
3-Mercapto-1, 2, 4-triazoles and N-acylated thiosemicarbazides as metallo-?-lactamase inhibitors.
Hussein W
Bioorganic & Medicinal Chemistry Letters, 22(1), 380-386 (2012)
G Scholz et al.
The Journal of biological chemistry, 264(8), 4318-4321 (1989-03-15)
4-Benzoylbenzoic acid inhibits pyridoxal kinase activity competitively with respect to pyridoxal. The Ki was determined to be 5 x 10(-5) M. Binding studies showed that 4-benzoylbenzoic acid bound to pyridoxal kinase at a 1:1 molar ratio and with a dissociation
Synthesis and luminescent properties of terbium complex containing 4-benzoylbenzoic acid for application in NUV-based LED.
Naiqun S
Journal of Rare Earths, 34(2), 130-136 (2016)
K M Defife et al.
Journal of biomaterials science. Polymer edition, 10(10), 1063-1074 (1999-12-11)
Amphiphilic chains of 4-benzoylbenzoic acid moieties and polymer were photochemically immobilized onto silicone rubber to ask whether the covalently coupled polymers would passivate the silicone rubber by inhibiting protein adsorption and subsequent cell adhesion and activation. Three groups of polymers
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service