A56655
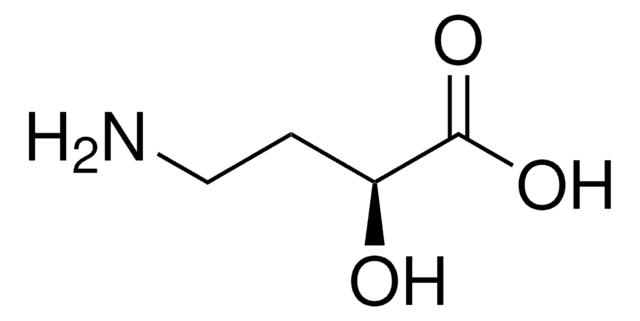
4-Amino-3-hydroxybutyric acid
98%
Synonym(s):
DL-γ-Amino-β-hydroxybutyric acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NCH2CH(OH)CH2CO2H
CAS Number:
Molecular Weight:
119.12
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder or crystals
reaction suitability
reaction type: solution phase peptide synthesis
color
white to yellow
mp
223 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
NCC(O)CC(O)=O
InChI
1S/C4H9NO3/c5-2-3(6)1-4(7)8/h3,6H,1-2,5H2,(H,7,8)
InChI key
YQGDEPYYFWUPGO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Yano et al.
Pharmacology, 40(4), 205-210 (1990-01-01)
Baclofen, gamma-amino-beta-hydroxybutyric acid (GABOB), calcium hopantenate (HOPA) and citicoline, which are clinically used for improving cerebral insufficiency, were tested for their effects on gastric acid secretion in the perfused stomach of urethane-anesthetized rats. These drugs were administered intravenously or subcutaneously.
Stereoselective analysis of racemic psychotropic compounds by HPLC on chiral stationary phase.
D F Smith et al.
Psychopharmacology, 89(3), 392-393 (1986-01-01)
M A Enero et al.
Clinical and experimental hypertension. Part A, Theory and practice, 10 Suppl 1, 331-337 (1988-01-01)
The cardiovascular effects of i.v. gamma-amino-beta-hydroxybutyric acid (GABOB) were investigated in rats anaesthetized with urethane. GABOB produced a dose-dependent hypotensive response. Treatment with GABA-A receptor antagonists prevented the GABOB response while the GABA stimulation by diazepam enhanced this response. The
Izumi Yamamoto et al.
ACS chemical neuroscience, 3(9), 665-673 (2012-09-29)
Designing potent and subtype-selective ligands with therapeutic value requires knowledge about how endogenous ligands interact with their binding site. 4-Amino-3-hydroxybutanoic acid (GABOB) is an endogenous ligand found in the central nervous system in mammals. It is a metabolic product of
Zhenjun Du et al.
Chirality, 16(8), 516-519 (2004-08-04)
Rh(2)(4S-MEOX)(4) and ethereal solvent are the best catalytic system for the enantioselective intramolecular C-H insertion of N-(2-benzyloxyethyl)-N-(tert-butyl)diazoacetamide 2. The highest enantiomeric excess obtained was 91%. A new route for the asymmetric synthesis of gamma-amino-beta-hydroxybutyric acid (GABOB) has been developed.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service