638021
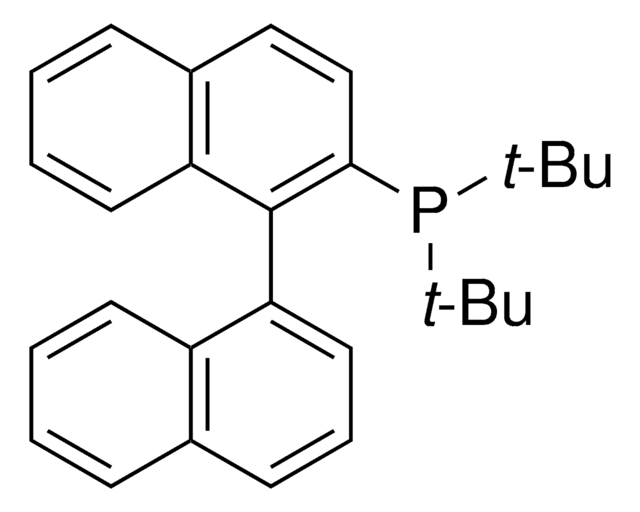
DavePhos
97%
Synonym(s):
2-Dicyclohexylphosphino-2′-(N,N-dimethylamino)biphenyl
About This Item
Recommended Products
Quality Level
Assay
97%
reaction suitability
reaction type: Cross Couplings
reagent type: ligand
reaction type: Arylations
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Hydroaminations
reagent type: ligand
reaction type: Kumada Coupling
reagent type: ligand
reaction type: Negishi Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
mp
121-124 °C (lit.)
functional group
phosphine
SMILES string
CN(C)c1ccccc1-c2ccccc2P(C3CCCCC3)C4CCCCC4
InChI
1S/C26H36NP/c1-27(2)25-19-11-9-17-23(25)24-18-10-12-20-26(24)28(21-13-5-3-6-14-21)22-15-7-4-8-16-22/h9-12,17-22H,3-8,13-16H2,1-2H3
InChI key
ZEMZPXWZVTUONV-UHFFFAOYSA-N
Application
- Palladium catalyzed sp3 arylation of 2-substituted-N-iminopyridinium ylides with different aryl chlorides to yield functionalized pyridines.
- Preparation of functionalized benzylic sulfones via palladium-catalyzed Negishi cross-coupling between alkyl sulfones and aryl halides.
- C-C Coupling of 3-haloquinolines with aryl sulfinates via palladium-catalyzed desulfitative arylation to form the corresponding biaryl compounds.
Learn more about Buchwald Phosphine Ligands
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Buchwald Phosphine Ligands
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Related Content
The Catalexis platform enhances catalysis by digitally optimizing catalyst selection to identify the most effective phosphine ligands for cross-coupling reactions.
The Catalexis platform enhances catalysis by digitally optimizing catalyst selection to identify the most effective phosphine ligands for cross-coupling reactions.
The Catalexis platform enhances catalysis by digitally optimizing catalyst selection to identify the most effective phosphine ligands for cross-coupling reactions.
The Catalexis platform enhances catalysis by digitally optimizing catalyst selection to identify the most effective phosphine ligands for cross-coupling reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service