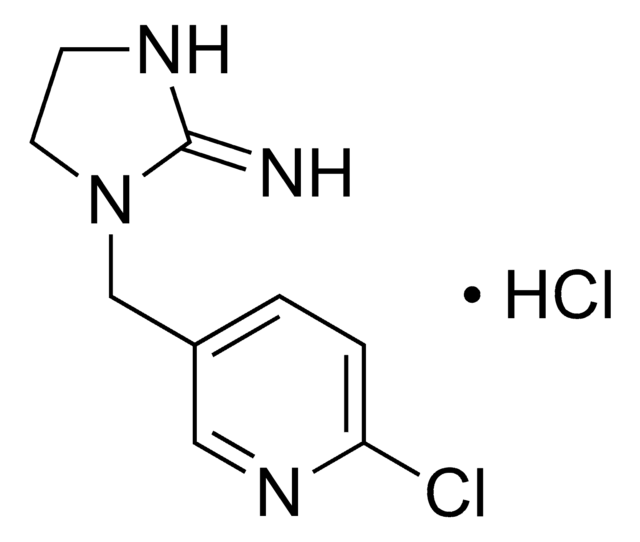
631175
Methyl 6-chloropyridine-3-carboxylate
98%
Synonym(s):
Methyl 6-chloronicotinate, Methyl 6-chloro-3-picolinate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H6ClNO2
CAS Number:
Molecular Weight:
171.58
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
86-90 °C (lit.)
functional group
chloro
ester
SMILES string
COC(=O)c1ccc(Cl)nc1
InChI
1S/C7H6ClNO2/c1-11-7(10)5-2-3-6(8)9-4-5/h2-4H,1H3
InChI key
RMEDXVIWDFLGES-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Methyl 6-chloropyridine-3-carboxylate can be used to synthesize fluoro substituted 6-phenylnicotinamide, which shows TRPV1 antagonist potency. It can also be used in the synthesis of retinoid x receptor (RXR) ligands, which have many clinical applications.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
"Replacing alkyl sulfonamide with aromatic sulfonamide in sulfonamide-type RXR agonists favors switch towards antagonist activity"
Morishita I-K, et al.
Bioorganic & Medicinal Chemistry, 19(03), 1001-1003 (2009)
"Design and synthesis of 6-phenylnicotinamide derivatives as antagonists of TRPV1"
Westaway.MS, et al.
Bioorganic & Medicinal Chemistry, 18(20), 5609-5613 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service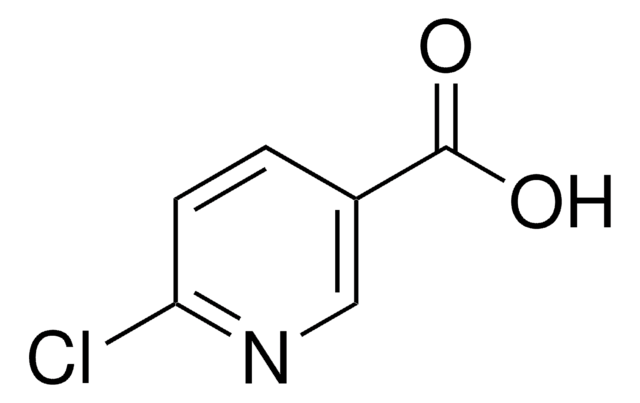
![(S)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol ≥99.0%](/deepweb/assets/sigmaaldrich/product/structures/201/440/11d18670-8609-4657-bb4b-af6c424f8791/640/11d18670-8609-4657-bb4b-af6c424f8791.png)