574589
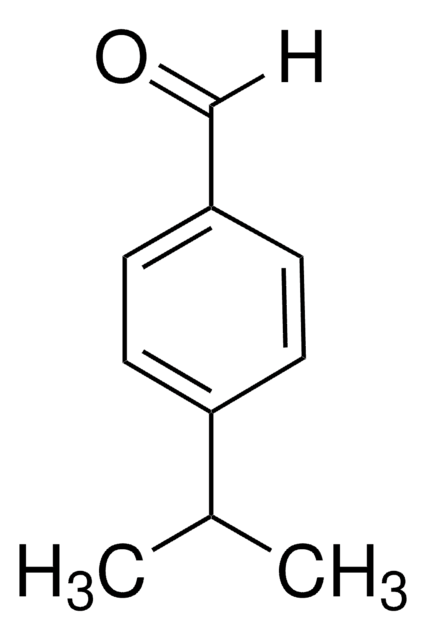
3,5-Di-tert-butylbenzaldehyde
97%
Synonym(s):
3,5-Di-tert-butyl-benzaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[(CH3)3C]2C6H3CHO
CAS Number:
Molecular Weight:
218.33
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
85-89 °C (lit.)
functional group
aldehyde
SMILES string
CC(C)(C)c1cc(C=O)cc(c1)C(C)(C)C
InChI
1S/C15H22O/c1-14(2,3)12-7-11(10-16)8-13(9-12)15(4,5)6/h7-10H,1-6H3
InChI key
BRUITYMDHWNCIG-UHFFFAOYSA-N
Application
3,5-Di-tert-butylbenzaldehyde may be used in the synthesis of the following:
- 5-p-pyridyl-15-(3,5-di-tert-butylphenyl)porphyrin via condensation reaction with 4-pyridinecarboxaldehyde and 2,2′-dipyrrylmethane
- 3,5-di-tert-butylphenyl-dipyrromethane via reaction with pyrrole in the presence of trifluoroacetic acid
- 3,5-di-tert-butyl-2-nitrobenzaldehyde via nitration reaction
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
"New ruthenium catalysts containing chiral Schiff bases for the asymmetric hydrogenation of acetophenone"
Karame I, et al.
Tetrahedron Asymmetry, 15(10), 1569-1581 (2004)
"Synthesis, conformational interconversion, and photophysics of tethered porphyrin?fullerene dyads with parachute topology"
Fazio.AM, et al.
Chemistry?A European Journal , 15(31), 7698-7705 (2009)
"A Self-Assembled Porphyrin Box from meso?meso-Linked Bis {5-p-pyridyl-15-(3, 5-di-octyloxyphenyl) porphyrinato zinc (II)}"
Tsuda A, et al.
Angewandte Chemie (Weinheim an der Bergstrasse, Germany), 114(15), 2941-2945 (2002)
Ying Jia et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(36), 12709-12714 (2015-08-01)
Yolk-shell nanoreactors with metal nanoparticle core and ultrathin porous polymer shells are effective catalysts for heterogeneous reactions. Polymer shells provide size-selectivity and improved reusability of catalyst. Nanocapsules with single-nanometer porous shells are prepared by vesicle-templated directed assembly. Metal nanoparticles are
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![4-[(Trimethylsilyl)ethynyl]benzaldehyde 97%](/deepweb/assets/sigmaaldrich/product/structures/350/679/378c15ce-18d5-4435-8a6c-f0974c6804ac/640/378c15ce-18d5-4435-8a6c-f0974c6804ac.png)