All Photos(1)
About This Item
Linear Formula:
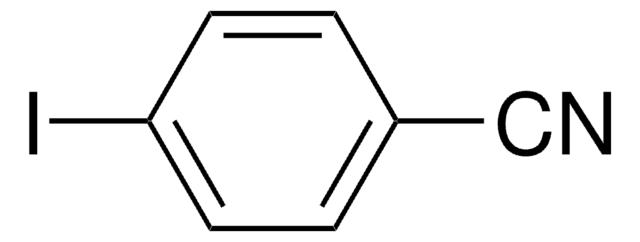
IC6H4CN
CAS Number:
Molecular Weight:
229.02
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
40-43 °C (lit.)
functional group
iodo
nitrile
SMILES string
Ic1cccc(c1)C#N
InChI
1S/C7H4IN/c8-7-3-1-2-6(4-7)5-9/h1-4H
InChI key
BGARPMGQRREXLN-UHFFFAOYSA-N
General description
3-Iodobenzonitrile is a halogenated aromatic nitrile. Its standard (ρ° = 0.1MPa) molar enthalpy of formation was determined by combustion calorimetry.
Application
3-Iodobenzonitrile may be used as a starting reagent in the synthesis of tetrachloroisophthalo-[14C]-nitrile (TCIN). It may also be used in the preparation of:
- 1-(3-iodophenyl)-3-{2-[4-(trifluoromethyl)-1-piperidinyl]ethyl}-2-imidazolidinone
- piperidine derivative
- chiral amino acid anilide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of tetrachloroisophthalo-[14C]-nitrile.
Davies PE.
Journal of Labelled Compounds & Radiopharmaceuticals, 21(3), 285-292 (1984)
Dominic P Affron et al.
European journal of organic chemistry, 2016(1), 139-149 (2016-02-16)
Saturated heterocycles, such as THFs, pyrrolidines, piperidines and THPs, are essential components of many biologically active compounds. Examples of C-H functionalization on these important ring systems remain scarce, especially at unactivated positions. Here we report the development of conditions for
Idriss Bennacef et al.
Bioorganic & medicinal chemistry letters, 19(17), 5056-5059 (2009-07-29)
Compound 1 is a potent and selective antagonist of the dopamine D(3) receptor. With the aim of developing a carbon-11 labeled ligand for the dopamine D(3) receptor, 1 was selected as a potential PET probe. [(11)C]1 was obtained by palladium
Synthesis of Chiral Amino Acid Anilides by Ligand-Free Copper-Catalyzed Selective N-Arylation of Amino Acid Amides
Dong J, et al.
Advanced Synthesis & Catalysis, 355(4), 692-696 (2013)
Thermodynamic and aromaticity studies for the assessment of the halogen? cyano interactions on Iodobenzonitrile.
Rocha IM, et al.
The Journal of Chemical Thermodynamics, 65, 204-212 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service