All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H7N3S
CAS Number:
Molecular Weight:
177.23
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
solid
mp
223-227 °C (lit.)
functional group
phenyl
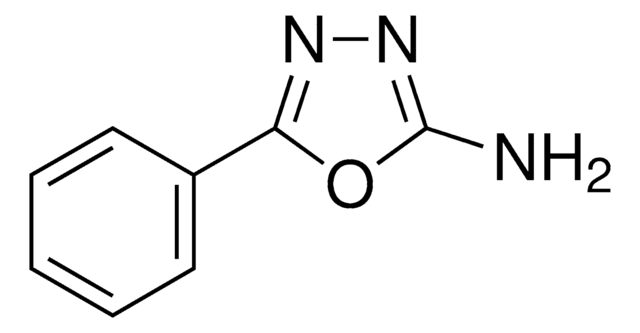
SMILES string
Nc1nnc(s1)-c2ccccc2
InChI
1S/C8H7N3S/c9-8-11-10-7(12-8)6-4-2-1-3-5-6/h1-5H,(H2,9,11)
InChI key
UHZHEOAEJRHUBW-UHFFFAOYSA-N
Related Categories
General description
2-Amino-5-phenyl-1,3,4-thiadiazole on condensation with benzaldehyde (SPT), 4-nitrobenzaldehyde (SNT), 4-methoxybenzaldehyde (SMT), 2-hydroxybenzaldehyde (SSTH) or 2-hydroxyacetophenone affords Schiff′s bases. Its molecular geometry and vibrational frequencies have been evaluated using the Hartree-Fock and density functional method (B3LYP). 2-Amino-5-phenyl-1,3,4-thiadiazole has been reported to inhibit the corrosion of mild steel in 0.5M H2SO4 and 1.0M HCl. Corrosion inhibition has been examined using potentiodynamic polarization and electrochemical impedance spectroscopy (EIS).
Application
2-Amino-5-phenyl-1,3,4-thiadiazole may be used for the synthesis of 1,3,4-thiadiazole derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis, characterization and antimicrobial activity of some carbamothioyl-1, 3, 4-thiadiazole derivatives.
Salih N, et al.
Synthesis, 4(2), 655-660 (2012)
A K Srivastava et al.
Bioinorganic chemistry and applications, 3(3-4), 289-297 (2008-03-28)
The reactions of bis(cyclopentadienyl)titanium(IV) dichloride with Schiff bases derived by condensing 2- amino-5-phenyl-1,3,4-thiadiazole with benzaldehyde (SPT), 4-nitrobenzaldehyde (SNT), 4-methoxybenzaldehyde (SMT), 2-hydroxybenzaldehyde (SSTH) or 2-hydroxyacetophenone (SATH) have been studied in refluxing tetrahydrofuran and complexes of types [Cp(2)TiCl(SB)]Cl (SB= SPT, SNT or
Y Atalay et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 64(1), 68-72 (2005-08-13)
The molecular geometry and vibrational frequencies of 2-amino-5-phenyl-1,3,4-thiadiazole (C8H7N3S) in the ground state has been calculated using the Hartree-Fock and density functional method (B3LYP) with 6-31G(d) basis set. The optimized geometric bond lengths and bond angles obtained by using HF
Experimental and molecular dynamics studies on corrosion inhibition of mild steel by 2-amino-5-phenyl-1, 3, 4-thiadiazole.
Tang Y, et al.
Corrosion Science, 52(1), 242-249 (2010)
Jingjing Zhang et al.
Carbohydrate polymers, 215, 108-118 (2019-04-15)
In the current study, five novel urea-functionalized chitosan derivatives were synthesized via condensation reactions of chloroacetyl chitosan (CTCS) with urea groups bearing nitrogen-containing heterocycles. In order to identify the structure characteristics of chitosan derivatives, FT-IR, 1H NMR spectroscopy, and elemental
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service