All Photos(1)
About This Item
Linear Formula:
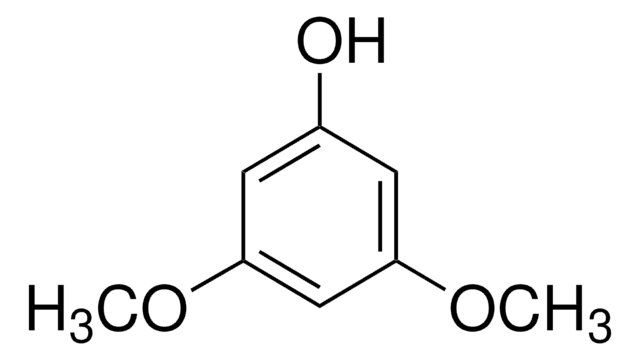
CH3C6H3(OCH3)2
CAS Number:
Molecular Weight:
152.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.522 (lit.)
bp
244 °C (lit.)
density
1.039 g/mL at 25 °C (lit.)
SMILES string
COc1cc(C)cc(OC)c1
InChI
1S/C9H12O2/c1-7-4-8(10-2)6-9(5-7)11-3/h4-6H,1-3H3
InChI key
RIZBLVRXRWHLFA-UHFFFAOYSA-N
General description
3,5-Dimethoxytoluene (DMT) is a methoxylated phenolic derivative. It is reported to be one of the main constituent of the floral volatiles in different rose varieties. It has been biosynthesized from orcinol by two successive methylation catalyzed by O-methyltransferases (OMTs). The features of its aerobic oxidation with metal/bromide catalysts have been investigated.
Application
3,5-Dimethoxytoluene (DMT) may be used in the synthesis of 3,5-dimethoxybenzoic acid by oxidation and 2-methoxy-6-methyl-1,4-benzoquinone by catalytic oxidation with hydrogen peroxide (H2O2)/methyltrioxorhenium (CH3ReO3) in dimethyl carbonate (DMC).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
215.6 °F - closed cup
Flash Point(C)
102 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Feng Zhang et al.
Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica, 29(5), 411-413 (2005-02-15)
To establish a method for GC fingerprint determination of the chemical constituents in Herba Asari. GC and GC-MS were used to optimize the fingerprint determination method, and identify the main peaks in the GC fingerprint. A preferable method for GC
Gabriel Scalliet et al.
Proceedings of the National Academy of Sciences of the United States of America, 105(15), 5927-5932 (2008-04-17)
The phenolic methyl ether 3,5-dimethoxytoluene (DMT) is a major scent compound of many modern rose varieties, and its fragrance participates in the characteristic "tea scent" that gave their name to Tea and Hybrid Tea roses. Among wild roses, phenolic methyl
The Unusual Characteristics of the Aerobic Oxidation of 3, 4-Dimethoxytoluene with Metal/Bromide Catalysts.
Partenheimer W.
Advanced Synthesis & Catalysis, 346(12), 1495-1500 (2004)
Studies in the biochemistry of micro-organisms: Synthesis of catenarin (1:4:5:7-tetrahydroxy-2-methylanthraquinone), a metabolic product of species of Helminthosporium.
W K Anslow et al.
The Biochemical journal, 35(8-9), 1006-1010 (1941-09-01)
Noa Lavid et al.
Plant physiology, 129(4), 1899-1907 (2002-08-15)
Rose (Rosa hybrida) flowers produce and emit a diverse array of volatiles, characteristic to their unique scent. One of the most prominent compounds in the floral volatiles of many rose varieties is the methoxylated phenolic derivative 3,5-dimethoxytoluene (orcinol dimethyl ether).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service