All Photos(2)
About This Item
Linear Formula:
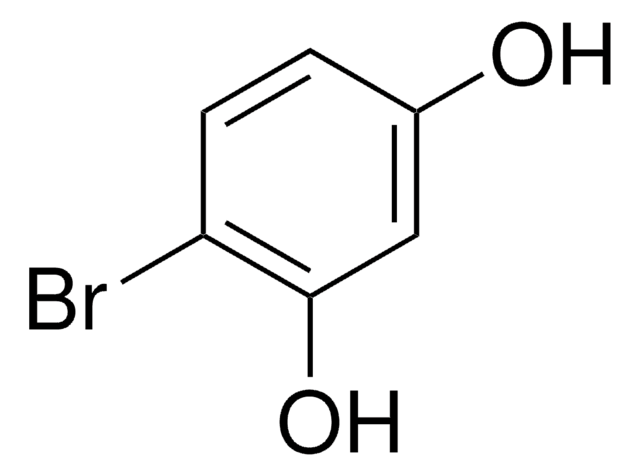
CH3C6H3-1,3-(OH)2
CAS Number:
Molecular Weight:
124.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
106-112 °C (lit.)
SMILES string
Cc1cc(O)cc(O)c1
InChI
1S/C7H8O2/c1-5-2-6(8)4-7(9)3-5/h2-4,8-9H,1H3
InChI key
OIPPWFOQEKKFEE-UHFFFAOYSA-N
Application
Orcinol can be used to synthesize:
- Orcinol-containing azacryptands for use in optical amplifiers and light-emitting devices.
- Ternary co-crystal with 4,4′-bipyridine.
- Low-density carbon aerogels in the presence of formaldehyde.
- PEG-orcinol coumarins with potent tyrosinase inhibitory activity.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Karen J Marsh et al.
Ecology, 87(8), 2103-2112 (2006-08-30)
Most herbivores eat more and survive better when they have access to a variety of foods. One explanation involves the detoxification of plant secondary metabolites (PSMs). By feeding from a variety of plants that contain different classes of PSMs, animals
Preparation of carbon aerogels from 5-methylresorcinol-formaldehyde gels
Perez-Caballero F, et al.
Microporous and Mesoporous Materials : The Official Journal of the International Zeolite Association, 108(1-3), 230-236 (2008)
Dibenzofurans from the marine sponge-derived ascomycete Super1F1-09.
Rateb ME, et al.
Botanica Marina, 53(6), 499-506 (2010)
Goutam Ghosh et al.
Pharmacognosy research, 7(1), 110-113 (2015-01-20)
Clerodendrum viscosum is commonly found in India and Bangladesh. Previously, various parts of this plant were reported for treatment of different types of diseases and there was no report on GC-Ms analysis. To analyze and characterize the phytochemical compounds of
Yong-qi Tian et al.
Natural product research, 29(9), 820-826 (2014-12-30)
A new citromycetin analogue, ascomycotin A (1), together with eight known compounds, wortmannilactone E (2), orcinol (3), orsellinic acid (4), isosclerone (5), (3R,4S)-( - )-4-hydroxymellein (6), diorcinol (7), chaetocyclinone B (8) and 2,5-dimethoxy-3,6-di(p-methoxypheny1)-1,4-benzoquinone (9), was isolated from the fungal strain Ascomycota sp.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service