417548
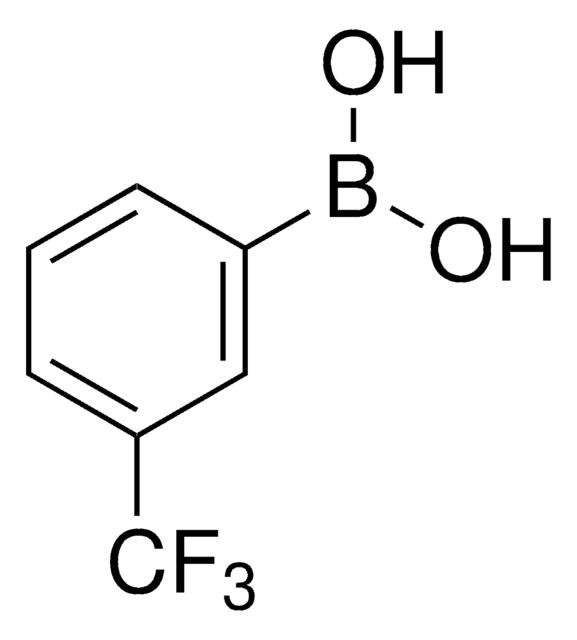
4-Chlorophenylboronic acid
95%
Synonym(s):
(p-Chlorophenyl)boronic acid, 4-Chlorobenzeneboronic acid, NSC 25408, p-Chlorobenzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
ClC6H4B(OH)2
CAS Number:
Molecular Weight:
156.37
Beilstein:
2936346
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
mp
284-289 °C (lit.)
functional group
chloro
SMILES string
OB(O)c1ccc(Cl)cc1
InChI
1S/C6H6BClO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4,9-10H
InChI key
CAYQIZIAYYNFCS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Chlorophenylboronic acid can be used as a reactant in:
It can also be used to prepare:
- Palladium-catalyzed direct arylation.
- Cyclopalladation.
- Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation.
- Copper-mediated ligandless aerobic fluoroalkylation.
- Pd-catalyzed arylative cyclization.
- Ruthenium catalyzed direct arylation.
- Ligand-free copper-catalyzed coupling reactions.
- Regioselective arylation and alkynylation by Suzuki-Miyaura and Sonogashira cross-coupling reactions.
It can also be used to prepare:
- Substituted diarylmethylidenefluorenes via Suzuki coupling reaction.
- Baclofen lactam by Suzuki coupling of a pyrrolinyl tosylate, followed by hydrogenation reaction.
- Palladium(II) thiocarboxamide complexes as Suzuki coupling catalysts.
- Biaryls by Suzuki reactions of aryl chlorides, bromides, and iodides with arylboronic acids.
Other Notes
Contains a varying amounts of anhydride
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Franco Furlani et al.
Carbohydrate polymers, 208, 451-456 (2019-01-20)
Developing synthetic materials able to mimic micro- and macrorheological properties of natural networks opens up to novel applications and concepts in materials science. The present contribution describes an active network based on a semi-synthetic polymer, a lactitol-bearing chitosan derivative (Chitlac)
Copper-Mediated Aerobic Fluoroalkylation of Arylboronic Acids with Fluoroalkyl Iodides at Room Temperature
Qi, Q.; Shen, Q.; Lu, L.
Journal of the American Chemical Society, 134, 648-6551 (2012)
A Double Suzuki Approach for Synthesis of Substituted Diarylmethylidenefluorenes
C. V. Ramana, et al.
Synlett, 1, 127-128 (2007)
Short synthesis of 4-aryl-3-pyrrolin-2-ones
Sarah J.P.Yoon-Miller, et al.
Tetrahedron Letters, 48, 827-830 (2007)
Immobilized palladium on surface-modified Fe3O4/SiO2 nanoparticles: as a magnetically separable and stable recyclable high-performance catalyst for Suzuki and Heck cross-coupling reactions
Du, Q.; et al.
Tetrahedron, 68, 3577-3584 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service