412244
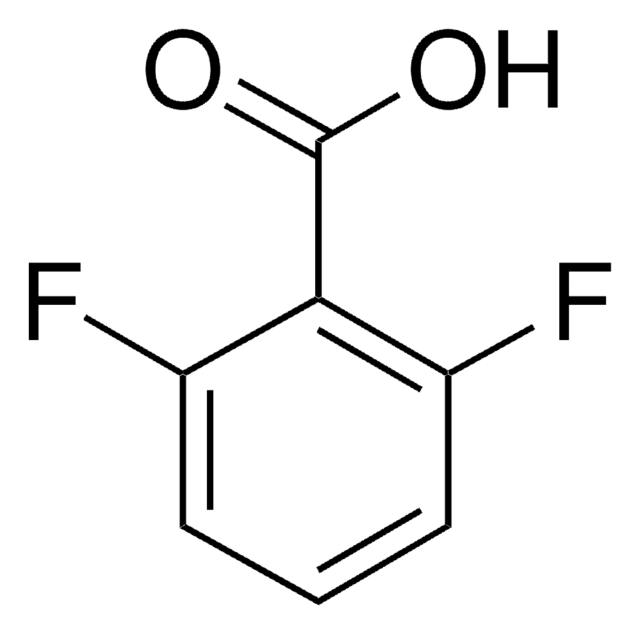
2-Fluorobenzoic acid
97%
Synonym(s):
o-Fluorobenzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
FC6H4CO2H
CAS Number:
Molecular Weight:
140.11
Beilstein:
971265
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
122-125 °C (lit.)
SMILES string
OC(=O)c1ccccc1F
InChI
1S/C7H5FO2/c8-6-4-2-1-3-5(6)7(9)10/h1-4H,(H,9,10)
InChI key
NSTREUWFTAOOKS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Fluorobenzoic acid may be employed in the preparation of zaragozic acid A analogs.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J Toretsky et al.
Nuclear medicine and biology, 31(6), 747-752 (2004-07-13)
The clinical response to antitumor therapy is measured using imaging, such as CT or MRI, 6-12 weeks following chemotherapy treatment. The images at that time reflect both tumor cell death and new growth. Therefore, the amount of tumor cell death
U Schennen et al.
Journal of bacteriology, 161(1), 321-325 (1985-01-01)
Three strains of anaerobically benzoate-degrading, denitrifying bacteria of the genus Pseudomonas were able to grow on 2-fluorobenzoate as the sole carbon and energy source. Fluoride ion release was stoichiometric, and the reduction of dissolved organic carbon indicated total degradation. Cells
S Y Lee et al.
Nuclear medicine and biology, 28(4), 391-395 (2001-06-08)
In vitro metabolism of acetylcholinesterase inhibitors containing 3-[(18)F]fluoromethylbenzyl- ([(18)F]1) and 4-[(18)F]fluorobenzyl-piperidine moieties ([(18)F]2) was studied and compared with the in vivo metabolism. Defluorination of the [(18)F]1 mainly occurred to generate [(18)F]fluoride ion both in vitro and in vivo. In contrast
Microbial degradation of synthetic organochlorine compounds.
K Motosugi et al.
Experientia, 39(11), 1214-1220 (1983-11-15)
M Begoña Osuna et al.
Water research, 42(14), 3857-3869 (2008-07-29)
Two up-flow fixed-bed reactors (UFBRs), inoculated with activated sludge and operated for 162 days, were fed 1mmolL(-1)d(-1) with two model halogenated compounds, 2-fluorobenzoate (2-FB) and dichloromethane (DCM). Expanded clay (EC) and granular activated carbon (GAC) were used as biofilm carrier.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service