410829
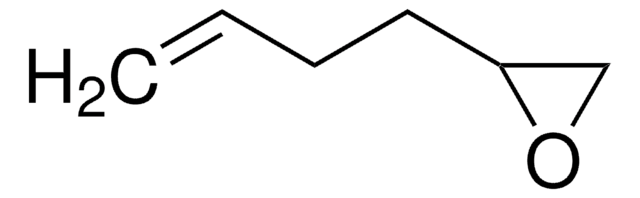
1,2-Epoxy-9-decene
96%
Synonym(s):
(7-Octenyl)oxirane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H18O
CAS Number:
Molecular Weight:
154.25
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
liquid
refractive index
n20/D 1.442 (lit.)
density
0.842 g/mL at 25 °C (lit.)
functional group
allyl
ether
SMILES string
C=CCCCCCCC1CO1
InChI
1S/C10H18O/c1-2-3-4-5-6-7-8-10-9-11-10/h2,10H,1,3-9H2
InChI key
FCZHJHKCOZGQJZ-UHFFFAOYSA-N
Related Categories
General description
1,2-Epoxy-9-decene is an unsaturated epoxide. Hydrogenation of the C=C bond of 1,2-epoxy-9-decene over palladium nanoparticles, namely PdOAc,N has been reported.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
172.4 °F - closed cup
Flash Point(C)
78 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Claire Jeanquartier et al.
Langmuir : the ACS journal of surfaces and colloids, 24(24), 13957-13961 (2009-04-11)
A versatile two-step method has been developed that allows linking of biomolecules covalently to hydrogen-terminated group-IV semiconductors by means of epoxy-alkenes. First, the terminal C==C double bond of the alkene forms a covalent bond with the silicon, germanium, or diamond
Yuan Lu et al.
Biomacromolecules, 14(10), 3589-3598 (2013-08-22)
A series of amphiphilic nitric oxide (NO)-releasing poly(amidoamine) (PAMAM) dendrimers with different exterior functionalities were synthesized by a ring-opening reaction between primary amines on the dendrimer and propylene oxide (PO), 1,2-epoxy-9-decene (ED), or a ratio of the two, followed by
Reactivity versus Stability of Oxiranes under Palladium-Catalyzed Reductive Conditions.
Thiery E, et al.
European Journal of Organic Chemistry, 2009(7), 961-985 (2009)
Yuan Lu et al.
Chemistry of materials : a publication of the American Chemical Society, 23(18), 4227-4233 (2011-11-05)
Structurally diverse secondary amine-functionalized poly(propylene imine) (PPI) dendrimers capable of tunable nitric oxide (NO) release were synthesized in a straightforward, one-step manner using ring-opening or conjugate-addition reactions with propylene oxide (PO), styrene oxide (SO), acrylonitrile (ACN), poly(ethylene glycol) methyl ether
Alexandra Shakun et al.
Polymers, 11(7) (2019-07-03)
Detonation nanodiamonds, also known as ultradispersed diamonds, possess versatile chemically active surfaces, which can be adjusted to improve their interaction with elastomers. Such improvements can result in decreased dielectric and viscous losses of the composites without compromising other in-rubber properties
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service