405000
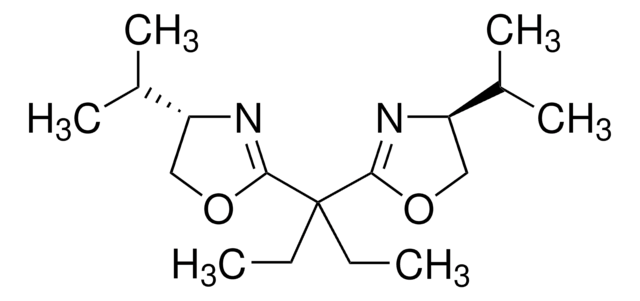
(−)-2,2′-Isopropylidenebis[(4S)-4-phenyl-2-oxazoline]
97%
Synonym(s):
(S,S)-2,2′-Isopropylidenebis(4-phenyl-2-oxazoline), (S,S)-2,2-Bis(4-phenyl-2-oxazolin-2-yl)propane
About This Item
Recommended Products
Quality Level
Assay
97%
optical activity
[α]20/D −160°, c = 1 in ethanol
bp
193 °C/0.03 mmHg (lit.)
mp
37-41 °C (lit.)
density
1 g/mL at 25 °C (lit.)
functional group
ether
phenyl
storage temp.
−20°C
SMILES string
CC(C)(C1=N[C@H](CO1)c2ccccc2)C3=N[C@H](CO3)c4ccccc4
InChI
1S/C21H22N2O2/c1-21(2,19-22-17(13-24-19)15-9-5-3-6-10-15)20-23-18(14-25-20)16-11-7-4-8-12-16/h3-12,17-18H,13-14H2,1-2H3/t17-,18-/m1/s1
InChI key
JTNVCJCSECAMLD-QZTJIDSGSA-N
Application
related product
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
BOX ligand complex enhances catalytic enantioselective aziridination of styrene, with phenyl substituents proving superior over t-Bu groups.
BOX ligand complex enhances catalytic enantioselective aziridination of styrene, with phenyl substituents proving superior over t-Bu groups.
BOX ligand complex enhances catalytic enantioselective aziridination of styrene, with phenyl substituents proving superior over t-Bu groups.
BOX ligand complex enhances catalytic enantioselective aziridination of styrene, with phenyl substituents proving superior over t-Bu groups.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![2,2′-Isopropylidenebis[(4S)-4-tert-butyl-2-oxazoline] 99%](/deepweb/assets/sigmaaldrich/product/structures/334/357/19788a81-5365-46fd-978b-6b98382b1117/640/19788a81-5365-46fd-978b-6b98382b1117.png)
![2,2′-Bis[(4S)-4-benzyl-2-oxazoline] 98%](/deepweb/assets/sigmaaldrich/product/structures/139/783/42da3c77-52af-401b-8525-35d96415e284/640/42da3c77-52af-401b-8525-35d96415e284.png)


![(+)-2,2′-Isopropylidenebis[(4R)-4-phenyl-2-oxazoline] 97%](/deepweb/assets/sigmaaldrich/product/structures/232/241/07f8baaa-0ba2-49e0-8ac2-f6d256fb2c84/640/07f8baaa-0ba2-49e0-8ac2-f6d256fb2c84.png)
![2,2′-Methylenebis[(4S)-4-phenyl-2-oxazoline] 97%](/deepweb/assets/sigmaaldrich/product/structures/255/350/4403d4f8-c973-4da7-a5b6-2e93d1eacb10/640/4403d4f8-c973-4da7-a5b6-2e93d1eacb10.png)
![[3aR-[2(3′aR*,8′aS*),3′aβ,8′aβ]]-(+)-2,2′-Methylenebis[3a,8a-dihydro-8H-indeno[1,2-d]oxazole] 98%](/deepweb/assets/sigmaaldrich/product/structures/134/031/294d2464-1571-4514-8e4c-c0cda1c1df7b/640/294d2464-1571-4514-8e4c-c0cda1c1df7b.png)

![2,6-Bis[(4S)-(−)-isopropyl-2-oxazolin-2-yl]pyridine 99%](/deepweb/assets/sigmaaldrich/product/structures/452/550/7e22a7c6-e84a-4741-af9a-e40f05d8061c/640/7e22a7c6-e84a-4741-af9a-e40f05d8061c.png)

![(4S)-(+)-Phenyl-α-[(4S)-phenyloxazolidin-2-ylidene]-2-oxazoline-2-acetonitrile 97%](/deepweb/assets/sigmaaldrich/product/structures/117/759/d4e6e882-8577-4dcf-84fe-506633ae811a/640/d4e6e882-8577-4dcf-84fe-506633ae811a.png)
![(4S)-(+)-4-[4-(tert-butyl)phenyl]-α-[(4S)-4-[4-(tert-butyl)phenyl]-2-oxazolidinylidene]-2-oxazolineacetonitrile 97%](/deepweb/assets/sigmaaldrich/product/structures/114/354/0740f93b-dd9c-48d5-a4be-92fe45387682/640/0740f93b-dd9c-48d5-a4be-92fe45387682.png)
![2,6-Bis[(3aS,8aR)-3a,8a-dihydro-8H-indeno[1,2-d]oxazolin-2-yl]pyridine](/deepweb/assets/sigmaaldrich/product/structures/302/311/68f9e406-0638-40fd-aafc-e36fe72b7ea8/640/68f9e406-0638-40fd-aafc-e36fe72b7ea8.png)
![2,6-Bis[(4R)-4-phenyl-2-oxazolinyl]pyridine 98%](/deepweb/assets/sigmaaldrich/product/structures/888/428/3be8313a-627a-4281-be35-306cc5da562a/640/3be8313a-627a-4281-be35-306cc5da562a.png)
![2,6-Bis[(4R)-(+)-isopropyl-2-oxazolin-2-yl]pyridine 99%](/deepweb/assets/sigmaaldrich/product/structures/349/609/8673c46e-368a-47a6-a9bd-52bbe13d490a/640/8673c46e-368a-47a6-a9bd-52bbe13d490a.png)