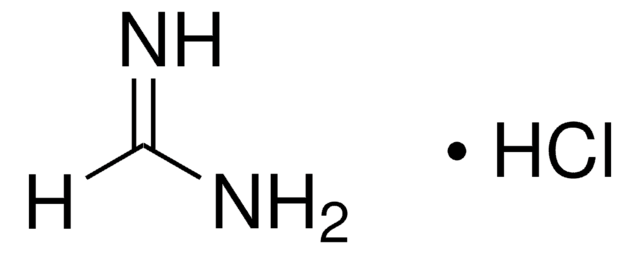396788
Ethyl formimidate hydrochloride
Synonym(s):
Ethyl methanimidate hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HC(=NH)OC2H5·HCl
CAS Number:
Molecular Weight:
109.55
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
mp
75 °C (dec.) (lit.)
functional group
ether
storage temp.
2-8°C
SMILES string
Cl.CCOC=N
InChI
1S/C3H7NO.ClH/c1-2-5-3-4;/h3-4H,2H2,1H3;1H
InChI key
JPUTTYRVDANTBN-UHFFFAOYSA-N
General description
Ethyl formimidate hydrochloride is a nitrogen containing organic building block. On heating it undergoes degradation to afford formamidine hydrochloride, ethyl formate and ethyl chloride.
Application
Ethyl formimidate hydrochloride may be used in the preparation of the following:
- 5-aminoimidazole-4-carboxylic acid α- and β-ribotides
- s-triazines
- 2,3:5,6-di-O-isopropylidene-α- and -β-5-amino-4-ethoxycarbonyl or -carbamoyl-imidazole D-mannofuranosides
- 5-amino-1-(2-pyridyl)imidazole
Reactant involved in the synthesis of biologically active molecules including:
Reactant involved in:
- Bredinin via amination of an acyclic precursor
- Amidine conjugates of the ornithine moiety of an antifungal
Reactant involved in:
- Intermolecular cyclization
- Mo-catalyzed asymmetric ring-closing metathesis for synthesis of cyclid amides and amines
- Synthesis of peptidic 1-cyanopyrrolidines
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Purines, pyrimidines, and imidazoles. XL. A new synthesis of a D-ribofuranosylamine derivative and its use in the synthesis of pyrimidine and imidazole nucleosides.
N J Cusack et al.
Journal of the Chemical Society. Perkin transactions 1, 16, 1720-1731 (1973-01-01)
Purines, pyrimidines, and imidazoles. Part XLI. Glycofuranosylamines derived from D-xylose, D-glucose, D-mannose, and L-rhamnose and their use in the synthesis of pyrimidine and imidazole nucleosides.
Cusack NJ, et al.
Journal of the Chemical Society. Perkin Transactions 1, 73-81 (1974)
Synthesis of the S-Triazine System. Iii. 1 Trimerization of Imidates.
Schaefer FC and Peters GA.
The Journal of Organic Chemistry, 26(8), 2771-2784 (1961)
Rhodd EH and von Richter V.
Chemistry of Organic Compounds: Pt. B. Aliphatic compounds, 551-551 (1951)
Purines, pyrimidines, and imidazoles part 54. Interconversion of some intermediates in the de novo biosynthesis of purine nucleotides.
Cusack NJ, et al.
Journal of the Chemical Society. Perkin Transactions 1, 2316-2321 (1980)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service