All Photos(1)
About This Item
Linear Formula:
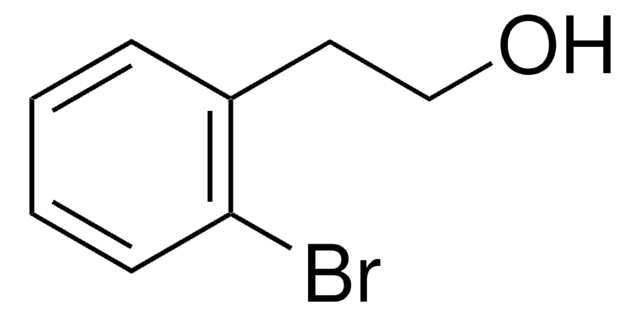
BrC6H4CH2CH2OH
CAS Number:
Molecular Weight:
201.06
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.5732 (lit.)
bp
107-110 °C/1 mmHg (lit.)
density
1.478 g/mL at 25 °C (lit.)
functional group
bromo
hydroxyl
SMILES string
OCCc1cccc(Br)c1
InChI
1S/C8H9BrO/c9-8-3-1-2-7(6-8)4-5-10/h1-3,6,10H,4-5H2
InChI key
PTTFLKHCSZSFOL-UHFFFAOYSA-N
Related Categories
General description
3-Bromophenethyl alcohol is a phenethyl alcohol derivative. Its enthalpy of vaporization at boiling point has been determined.
Application
3-Bromophenethyl alcohol may be used as a starting material in the synthesis of its mesylate derivative (m-Br-C6H4CH2CH2OMs) by treatment with mesyl chloride.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yaws CL.
Thermophysical Properties of Chemicals and Hydrocarbons, 466-466 (2008)
Annie Tam et al.
Bioorganic & medicinal chemistry, 17(3), 1055-1063 (2008-03-04)
The traceless Staudinger ligation can be mediated by phosphinothiols under physiological conditions. Proximal positive charges are necessary to achieve that transformation, presumably because those charges discourage protonation of the key iminophosphorane intermediate. Here, a series of cationic phosphinothiols is used
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service