All Photos(2)
About This Item
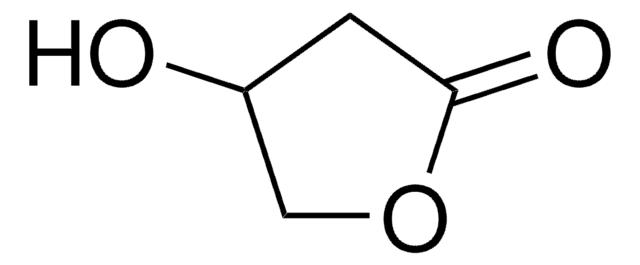
Empirical Formula (Hill Notation):
C5H6O3
CAS Number:
Molecular Weight:
114.10
Beilstein:
3537455
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
optical activity
[α]20/D −144°, c = 1 in H2O
mp
41-43 °C (lit.)
functional group
ester
hydroxyl
storage temp.
2-8°C
SMILES string
OC[C@H]1OC(=O)C=C1
InChI
1S/C5H6O3/c6-3-4-1-2-5(7)8-4/h1-2,4,6H,3H2/t4-/m0/s1
InChI key
AWNLUIGMHSSXHB-BYPYZUCNSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
(S)-(−)-5-Hydroxymethyl-2(5H)-furanone can be used as a starting material in the preparation of:
- Partially saturated heterocycles via a diastereoselective ring chain formation.
- A natural product named (+)-muscarine.
- 3′-Ethynylthymidine as a possible antiviral agent.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of (+)-muscarine from (S)-(-)-5-hydroxymethyl-2 (5H)-furanone
Kang KH, et al.
Tetrahedron Letters, 41(42), 8137-8140 (2000)
Synthesis of 3′-ethynylthymidine, 3′-vinylthymidine and 3′-bromovinylthymidine as potential antiviral agents
Sahlberg C
Tetrahedron Letters, 33(5), 679-682 (1992)
Bohrisch, J. et al.
Tetrahedron Letters, 34, 2749-2749 (1993)
Highly diastereoselective ring chain transformation of butonolides to 5-(a-hydroxyalkyl) pyrazolidin-3-ones
Bohrisch J, et al.
Tetrahedron Letters, 34(17), 2749-2752 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service