295809
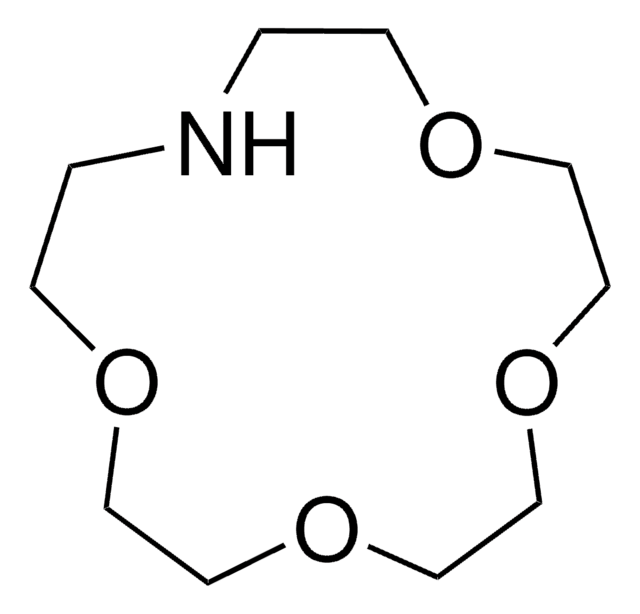
1,4,10,13-Tetraoxa-7,16-diazacyclooctadecane
≥96%
Synonym(s):
1,10-Diaza-18-crown-6
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H26N2O4
CAS Number:
Molecular Weight:
262.35
Beilstein:
609764
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥96%
form
solid
mp
111-114 °C (lit.)
functional group
ether
SMILES string
C1COCCOCCNCCOCCOCCN1
InChI
1S/C12H26N2O4/c1-5-15-9-10-17-7-3-14-4-8-18-12-11-16-6-2-13-1/h13-14H,1-12H2
InChI key
NLMDJJTUQPXZFG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1,4,10,13-Tetraoxa-7,16-diazacyclooctadecane is a crown ether, which can be used:
- In the spectroscopic studies of its complex-forming reaction with iodine.
- To prepare the ligand 7,16-bis(5-t-butyl-2-hydroxybenzyl)-1,4,10,13-tetraoxa-7,16-diazacyclooctadecane.
- As a ligand in the study of zirconium facilitated hydrolysis of a dipeptide at neutral pH.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Spectroscopic studies of the reaction of iodine with the mixed oxygen-nitrogen cyclic base 1, 4, 10, 13-tetraoxa-7, 16-diazacyclooctadecane
Nour E
Spectrochimica Acta Part A: Molecular Spectroscopy, 47(6), 743-747 (1991)
Tuning Zr (IV)-assisted peptide hydrolysis at near-neutral pH
Kassai M and Grant KB
Inorganic Chemistry Communications, 11(5), 521-525 (2008)
Syntheses and crystal structures of copper complexes of 7, 16-bis (5-t-butyl-2-hydroxybenzyl)-1, 4, 10, 13-tetraoxa-7, 16-diazacyclooctadecane
Ma S, et al.
Polyhedron, 22(25-26), 3249-3253 (2003)
L P Varga et al.
International journal of radiation biology, 66(4), 399-405 (1994-10-01)
To date, there has been no effective therapy to counter incorporated radionuclides of strontium. In an endeavour to solve this problem, we have synthesized and evaluated various N,N'-disubstituted derivatives of 1,4,10,13-tetraoxa-7,16-diaza-cyclooctadecane(crypt and 2.2) for their ability to mobilize 85Sr2+. These
Fran Supek et al.
European journal of medicinal chemistry, 46(8), 3444-3454 (2011-06-02)
18-crown-6 ethers are known to exert their biological activity by transporting K(+) ions across cell membranes. Using non-linear Support Vector Machines regression, we searched for structural features that influence antiproliferative activity in a diverse set of 19 known oxa-, monoaza-
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane 98%](/deepweb/assets/sigmaaldrich/product/structures/189/812/8a6555e5-8de6-4236-865f-19339cee3634/640/8a6555e5-8de6-4236-865f-19339cee3634.png)