All Photos(1)
About This Item
Empirical Formula (Hill Notation):
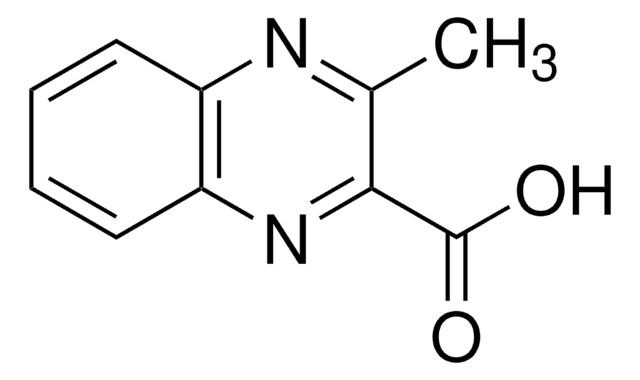
C9H6N2O2
CAS Number:
Molecular Weight:
174.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
208 °C (dec.) (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)c1cnc2ccccc2n1
InChI
1S/C9H6N2O2/c12-9(13)8-5-10-6-3-1-2-4-7(6)11-8/h1-5H,(H,12,13)
InChI key
UPUZGXILYFKSGE-UHFFFAOYSA-N
General description
Linear and Freundlich adsorption isotherm coefficient of 2-quinoxalinecarboxylic acid has been evaluated.
Application
2-Quinoxalinecarboxylic acid has been used in the preparation of:
- N-(2-quinoxaloyl)-α-amino acids
- bisquinoxaloyl (bisquinoxalinecarbonyl) derivatives
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M D Rose et al.
Food additives and contaminants, 12(2), 177-183 (1995-03-01)
A method for the determination of residues of quinoxaline-2-carboxylic acid (QCA), the major metabolite of carbadox, in pig kidney has been developed. Tissue samples were subjected to alkaline hydrolysis, liquid-liquid extractions, ion-exchange chromatography and further extraction to concentrate the analyte
N Prabavathi et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 92, 325-335 (2012-03-27)
The FTIR and FT-Raman spectra of 2-quinoxaline carboxylic acid (2-QCA) has been recorded in the region 4000-450 and 4000-100 cm(-1), respectively. The conformational analysis, optimized geometry, frequency and intensity of the vibrational bands of 2-QCA were obtained by the density
Joshua A Hagen et al.
Sensors (Basel, Switzerland), 11(7), 6645-6655 (2011-12-14)
Zinc oxide field effect transistors (ZnO-FET), covalently functionalized with single stranded DNA aptamers, provide a highly selective platform for label-free small molecule sensing. The nanostructured surface morphology of ZnO provides high sensitivity and room temperature deposition allows for a wide
Lingli Huang et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 874(1-2), 7-14 (2008-10-08)
Cyadox (CYX) is an antimicrobial growth-promoter of the quinoxalines. It is highly effective on improving growth and feed conversion of chicken with little toxicity. For food safety concerns, HPLC-UV methods were developed for the sequential determination of CYX and its
D Peng et al.
Food additives & contaminants. Part A, Chemistry, analysis, control, exposure & risk assessment, 28(11), 1524-1533 (2011-08-11)
The feed drug additive carbadox is a suspected carcinogen and mutagen. To monitor effectively residues of carbadox in the edible tissues of food-producing animals, an indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) to detect quinoxaline-2-carboxylic acid, the marker residue of carbadox
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service