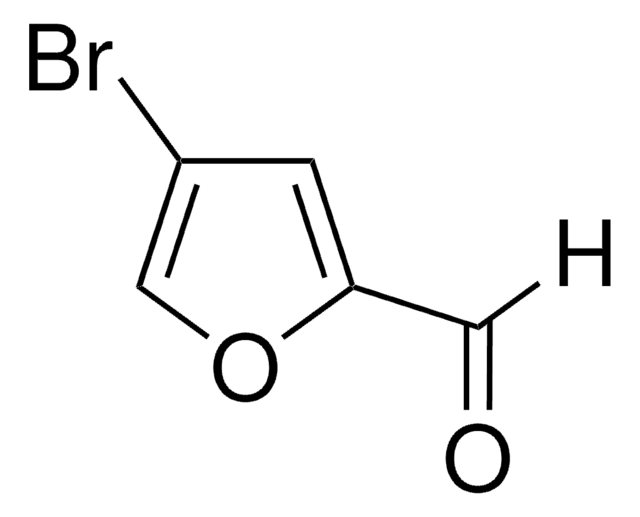
278866
3-Furancarboxaldehyde
≥97%
Synonym(s):
3-Furaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H4O2
CAS Number:
Molecular Weight:
96.08
Beilstein:
105852
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97%
form
liquid
refractive index
n20/D 1.493 (lit.)
bp
144 °C/732 mmHg (lit.)
density
1.111 g/mL at 25 °C (lit.)
functional group
aldehyde
storage temp.
2-8°C
SMILES string
O=Cc1ccoc1
InChI
1S/C5H4O2/c6-3-5-1-2-7-4-5/h1-4H
InChI key
AZVSIHIBYRHSLB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Furancarboxaldehyde undergoes photochemical reaction with benzaldehyde to yield oxetane derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
118.4 °F - closed cup
Flash Point(C)
48 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Maurizio D'Auria et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 9(8), 1134-1138 (2010-06-22)
The photochemical reaction of 2-substituted heterocyclic aldehydes with furan gave the corresponding exo oxetane derivatives through the excited triplet state. However, in situ the oxetane derivatives were converted through a metathesis reaction into the corresponding Z,E-butadienyl formate derivatives. On the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service












![Benzo[b]thiophene-2-carboxaldehyde 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)