252131
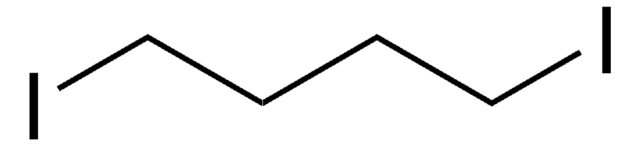
1,5-Diiodopentane
97%, contains copper as stabilizer
Synonym(s):
α,ψ-Diiodopentane, (±)-Hexane-1,2-diol, Pentamethylene diiodide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
I(CH2)5I
CAS Number:
Molecular Weight:
323.94
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
contains
copper as stabilizer
refractive index
n20/D 1.6002 (lit.)
bp
101-102 °C/3 mmHg (lit.)
density
2.177 g/mL at 25 °C (lit.)
functional group
iodo
SMILES string
ICCCCCI
InChI
1S/C5H10I2/c6-4-2-1-3-5-7/h1-5H2
InChI key
IAEOYUUPFYJXHN-UHFFFAOYSA-N
Related Categories
General description
Electrochemical reduction of 1,5-diiodopentane at carbon electrodes in DMF containing tetramethylammonium perchlorate has been studied by cyclic voltammetry and controlled-potential electrolysis.
Application
1,5-Diiodopentane has been used:
- as crosslinking reagent, to investigate the cross-linking layer-by-layer films of bioreducible poly(2-dimethylaminoethyl methacrylate) and DNA
- in preparation of (iodoalkylaminocarbene)tungsten complexes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Reactions of a-lithio (aminocarbene) tungsten anions with diiodoalkanes: Synthesis of (?-bis (aminocarbene)) ditungsten complexes.
Macomber DW and Madhukar P.
Journal of Organometallic Chemistry, 433(3), 279-285 (1992)
Cross-linked bioreducible layer-by-layer films for increased cell adhesion and transgene expression.
Jenifer Blacklock et al.
The journal of physical chemistry. B, 114(16), 5283-5291 (2010-04-08)
The effect of cross-linking layer-by-layer (LbL) films consisting of bioreducible poly(2-dimethylaminoethyl methacrylate) (rPDMAEMA) and DNA is examined with regard to rigidity, biodegradability, cell adhesion, and transfection activity using 1,5-diiodopentane (DIP) cross-linker. DIP chemically reacts with the tertiary amines of rPDMAEMA
Electrochemical Reduction of 1, 5-Dihalopentanes at Carbon Cathodes in Dimethylformamide.
Pritts WA and Peters DG.
Journal of the Electrochemical Society, 141(12), 3318-3324 (1994)
Ian C Watson et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 25(41), 9678-9690 (2019-05-16)
New N-heterocyclic olefins (NHOs) are described with functionalization on the ligand heterocyclic backbone and terminal alkylidene positions. Various PdII -NHO complexes have been formed and their use as pre-catalysts in Buchwald-Hartwig aminations was explored. The most active system for catalytic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service