All Photos(1)
About This Item
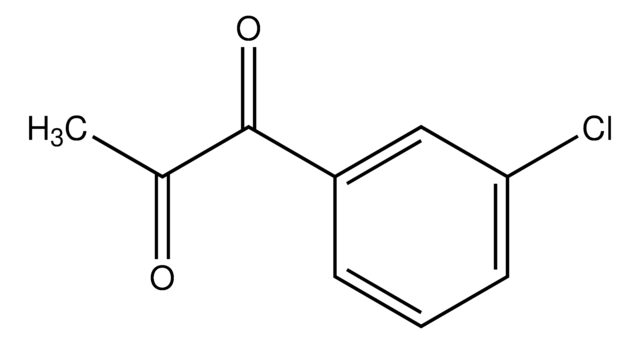
Linear Formula:
ClC6H4COC2H5
CAS Number:
Molecular Weight:
168.62
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
124 °C/14 mmHg (lit.)
mp
45-47 °C (lit.)
functional group
chloro
ketone
SMILES string
CCC(=O)c1cccc(Cl)c1
InChI
1S/C9H9ClO/c1-2-9(11)7-4-3-5-8(10)6-7/h3-6H,2H2,1H3
InChI key
PQWGFUFROKIJBO-UHFFFAOYSA-N
Related Categories
General description
Influence of solvents and temperature on the yield and enantioselectivity of the phenylation of 3′-chloropropiophenone has been investigated.
Application
3′-Chloropropiophenone can be used as a reactant to synthesize:
- (S)-3-chloro-1-phenylpropanol via bio-catalyzed asymmetric reduction method.
- 1-(3-Chlorophenyl)-1-phenyl-1-propanol by phenylation with diphenylzinc in the presence of dihydroxy bis(sulfonamide) ligand.
- (S)-Dapoxetine, a selective serotonin reuptake inhibitor.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Asymmetric reduction of (S)-3-chloro-1-phenylpropanol from 3-chloropropiophenone by preheated immobilized Candida utilis
Yang Gen-Sheng, et al.
Biotechnology Letters, 12, 1879-1883 (2009)
Asymmetric total synthesis of (S)-dapoxetine
Kim Sun Joo, et al.
Tetrahedron Letters, 53(28), 3680-3682 (2012)
Enhancing asymmetric reduction of 3-chloropropiophenone with immobilized Acetobacter sp. CCTCC M209061 cells by using deep eutectic solvents as cosolvents
Xu Pei, et al.
ACS sustainable chemistry & engineering, 3(4), 718-724 (2015)
Celina García et al.
Organic letters, 5(20), 3641-3644 (2003-09-26)
[reaction: see text] The catalytic asymmetric addition of phenyl groups from diphenylzinc to ketones is reported. The catalyst, generated from a dihydroxy bis(sulfonamide) ligand and titanium tetraisopropoxide, gives good to excellent enantioselectivities with a range of substrates.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service