All Photos(2)
About This Item
Linear Formula:
(CH3)2CHSO2Cl
CAS Number:
Molecular Weight:
142.60
Beilstein:
1747497
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
4 (vs air)
Quality Level
vapor pressure
15.5 mmHg ( 25 °C)
Assay
97%
form
liquid
autoignition temp.
865 °F
refractive index
n20/D 1.453 (lit.)
bp
74-75 °C/19 mmHg (lit.)
density
1.27 g/mL at 25 °C (lit.)
SMILES string
CC(C)S(Cl)(=O)=O
InChI
1S/C3H7ClO2S/c1-3(2)7(4,5)6/h3H,1-2H3
InChI key
DRINJBFRTLBHNF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The solvolysis of 2-propanesulfonyl chloride by an addition-elimination (association-dissociation) pathway was studied.
Application
2-Propanesulfonyl chloride (isopropylsulfonyl chloride) can be used as a reactant to prepare:
- 1-Isopropylsulfonyl-2-amine benzimidazole by reacting with 2-aminobenzimidazole via N-sulfonylation reaction in the presence of a base.
- Bis(isopropylsulfonyl)disulfide, a sulfurizing agent, used to synthesize phosphorothioate oligonucleotide analogs.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
186.8 °F - closed cup
Flash Point(C)
86 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Identification of 1-isopropylsulfonyl-2-amine benzimidazoles as a new class of inhibitors of hepatitis B virus
Li, Yun-Fei, et al.
European Journal of Medicinal Chemistry, 42, 1358-1364 (2007)
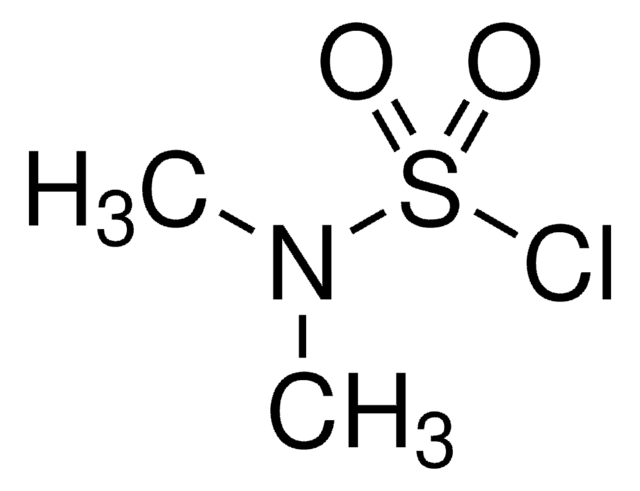
Rate and product studies in the solvolyses of N,N-dimethylsulfamoyl and 2-propanesulfonyl chlorides.
Dennis N Kevill et al.
Organic & biomolecular chemistry, 4(8), 1580-1586 (2006-04-11)
Contrary to earlier suggestions of an S(N)1 pathway for solvolyses of N,N-dimethylsulfamoyl chloride (1), an extended Grunwald-Winstein equation treatment of the specific rates of solvolysis in 32 solvents shows an appreciable sensitivity towards changes in both solvent nucleophilicity and solvent
New efficient sulfurizing reagents for the preparation of oligodeoxyribonucleotide phosphorothioate analogues
Efimov Vladimir A, et al.
Nucleic Acids Research, 23, 4029-4033 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service